HTML
-
It has been over a century since the first techniques utilizing cell culture in vitro were reported. In 1907, Ross Harrison isolated and cultured a small piece of living frog embryonic tissue in a hanging drop1. Later, scientists developed the classic 2D monolayer cell culture system. However, they were not able to mimic in vivo states on plastic Petri dishes because tissue-specific architecture, cell-cell interactions, and cell-matrix interactions are lost in the process. The obvious drawbacks of 2D cell culture have led scientists to develop 3D cell culture techniques. As early as 1972, the first 3D cell culture system in vitro was reported using collagen as the extracellular scaffold2. Bard and Elsdale compared the differences between cell morphology, growth, and behaviour under 2D and 3D cell culture conditions and revealed the striking similarity of cells in the 3D culture system and in vivo. It is well accepted that 3D cell culture technologies are becoming essential for drug screening, tissue engineering, and other translational research.
The extracellular matrix (ECM) plays a pivotal role in 3D cell culture, and many natural and synthetic scaffolds provide various culture conditions that have different desirable properties, including stiffness, biodegradability, porosity, and cytocompatibility3. Matrigel is among the most ubiquitous natural scaffolds in 3D cell culture, with an average elastic modulus of ~650 Pa4. It is a basement membrane matrix extracted from Engelbreth-Holm-Swarm murine sarcoma, first developed in 19775. Matrigel provides the desired mechanical properties, sufficient cytocompatibility, biomimicry to native ECM, and the ability to promote cell adhesion and differentiation across many cell types6.
However, the applicability of Matrigel is severely limited by the variability in its composition and the presence of xenogeneic contaminants. Therefore, chemically defined native-based or synthetic-based xeno-free alternatives are in great demand. The wide acceptance of Matrigel in the last few decades may partially be attributed to the lack of other scaffolds that are easy to use and versatile for culturing different types of cells. Recently, some synthetic materials have shown comparable or superior outcomes in 3D cell culture, tissue engineering, and organoid-based studies of both development and diseases7–10.
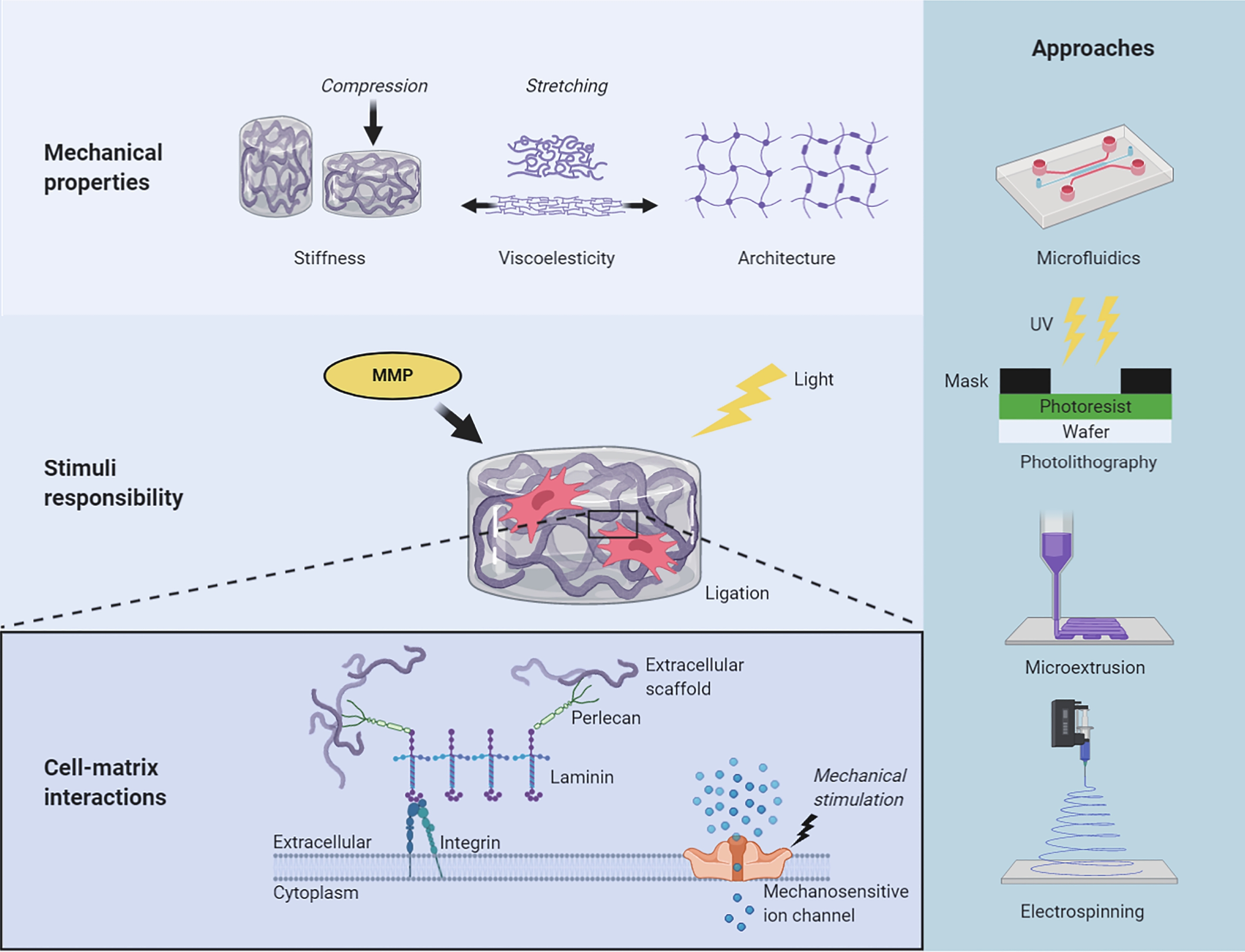
In this review, we begin by discussing the importance of three endowed and desired properties of extracellular scaffold design: mechanical properties, cell-matrix interactions, and spatiotemporally “programmable” stimuli responsibility. We then briefly summarise the engineering approaches that take advantage of these properties, such as microfluidics, electrospinning, photolithography, and microextrusion (Fig. 1). We propose the term microtissue engineering, which refers to the techniques that formulate small-scale (e.g. smaller than a few millimetres) artificial tissue structures with microscale precision. We refer to ultra-softness as the modulus range below a few kilopascal, which is the typical stiffness of many soft tissues, including the brain, lung, liver, and skin, as well as their reconstituted extracellular matrices. Finally, we discuss the current impediments to rational design and manufacturing of extracellular scaffolds with microscale precision and provide our perspective on the future of engineering scaffolds for cell culture applications and translational research.
-
To understand the different behaviours of cells in 2D and 3D culture conditions, biologists were intently focused on deciphering signal transduction pathways, until they realised that not only receptor binding but also physical entanglements of cells and their surrounding scaffolds would also alter critical cellular events11. Pelham et al. demonstrated that substrate stiffness could affect cell adhesion, morphology, and locomotion by using polyacrylamide hydrogels with varying elastic moduli as the supporting scaffolds12. Since then, many studies have found that ECM stiffness affects cell behaviours from a range of dimensions and plays a key role in regulating development, homeostasis, regenerative processes, disease progression, and cell response to drugs. However, tissues are not purely elastic materials, like rubber or a spring. They exhibit a time-dependent mechanical response and dissipate a fraction of the energy that deforms them, a property called viscoelasticity or poroelasticity, depending on the molecular mechanism13. Architectural features and external forces have also been reported to affect various aspects of cell behaviour. These findings improve the current stiffness-centric view of cell-matrix mechanotransduction and challenging in vitro modelling.
The stiffness of the scaffold, or as referred in biomaterial design, the elasticity of the material, has been of great concern once the subject is established. Cell lineage proliferation and differentiation, especially pluripotent stem cells (e.g. embryonic stem cells [ESCs] and induced pluripotent stem cells [iPSCs]), are highly sensitive to scaffold stiffness14–16. Directed stem-cell differentiation may be achieved by reconstituting the stiffness of the natural tissue environment with an engineered scaffold: soft scaffolds that mimic the elastic modulus of the brain (0.1–1 kPa) can be neurogenic; scaffolds of intermediate stiffness that mimic skeletal muscle (8–17 kPa) can be myogenic; and rigid scaffolds that mimic bone (25–40 kPa) are osteogenic17.
Natural materials, such as collagen and Matrigel, may have an inherent inability to decouple their biochemical and mechanical properties. These biological materials are not only very soft (Young’s modulus E < 1 kPa) but are difficult to characterise. They may suffer spatiotemporal heterogeneities in both biological and mechanical properties because of their complex composition or poorly controlled solidifying processes. The uses in both basic research and translation have been hampered by batch-to-batch inconsistency and lack of tunability. Murphy et al. tested the elastic modulus of Matrigel using atomic force microscopy (AFM). They reported an average modulus of approximately 450 Pa18. However, they observed a static measurement deviation of 840 Pa in the same sample, which could be attributed to the intrinsic inhomogeneity in spatial composition and the probed micromorphology in the measurement. Mechanical imaging interferometry was applied to obtain point mechanics of the surface layers of the Matrigel4. The median value of 650 Pa was consistent with bulk measurements; however, on the microscale, the films were heterogeneous and contained regions distinctly stiffer than their surroundings, which varied within the range of 1−2 kPa. Therefore, mechanically tunable and chemically defined synthetic alternatives have been proposed to overcome the limitations of Matrigel.
Viscoelasticity, a historically ignored property in biomaterial design, is accepted as another key feature of biomaterials13. Living cell activities in vivo and 3D cell culture in vitro are dynamic processes, including cell-matrix interactions, cell-cell interactions, and time-dependent scaffold manners, such as creeping, stress loading, and relaxation. Viscoelasticity is a near-universal feature of living tissues and native ECMs, but not of some widely used synthetic materials, such as polyacrylamide (PAM) hydrogels and polydimethylsiloxane (PDMS) elastomers, which are linearly elastic. In linear elastic materials, stress is linearly related to strain for small strains, with no loss of mechanical energy and reversible deformations. In comparison, viscoelastic materials exhibit stress relaxation in response to constant deformation and increased strain, or creep, in response to constant stress.
Matrix viscoelasticity regulates cell fate in 3D cultures. For example, stress-relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fibre remodelling, and focal adhesion formation19. Faster stress relaxation and increased loss promote cell cycle progression and completion of mitosis in single cancer cells and fibroblasts, as well as osteogenic differentiation of mesenchymal stem cells (MSCs)20. Darnell et al. used the RNA-seq method to reveal the transcriptional responses of cells in 3D culture to stress relaxation, matrix stiffness, and adhesion ligand density. They exhibited substantial independent effects and coupling among these properties, demonstrating clear cell-type and context-dependent viscoelasticity sensing16.
Microtissue engineering is fundamental for organoid manufacturing. Architectural features, including geometry, porosity, and topology, as well as external forces, determine the tissue shape and growth kinetics. Geometry is a well-known factor that controls cell proliferation and organoid formation under 3D culture conditions. Lawlor et al. changed kidney organoid length while maintaining the same absolute number of starting cells. The results showed that the width-to-length ratio changes in organoid conformation affected organoid morphology, increasing the abundance of nephrons, conformation, and maturity of epithelial structures23 (Fig. 2a–2c). Porosity, including the scaffold pore distribution, is another architectural factor that critically determines the growth rate and nature of vascular sprout branching, while stromal cells promote hypoxia signalling and secrete chemokines to promote this outcome24. External forces after gelation are influenced by different mechanical cues from the internal stiffness of the scaffold. Li et al. showed that volumetric compression regulates the growth of intestinal organoids by modifying intracellular crowding and elevating Wnt/β-catenin signalling22 (Fig 2d, 2e).
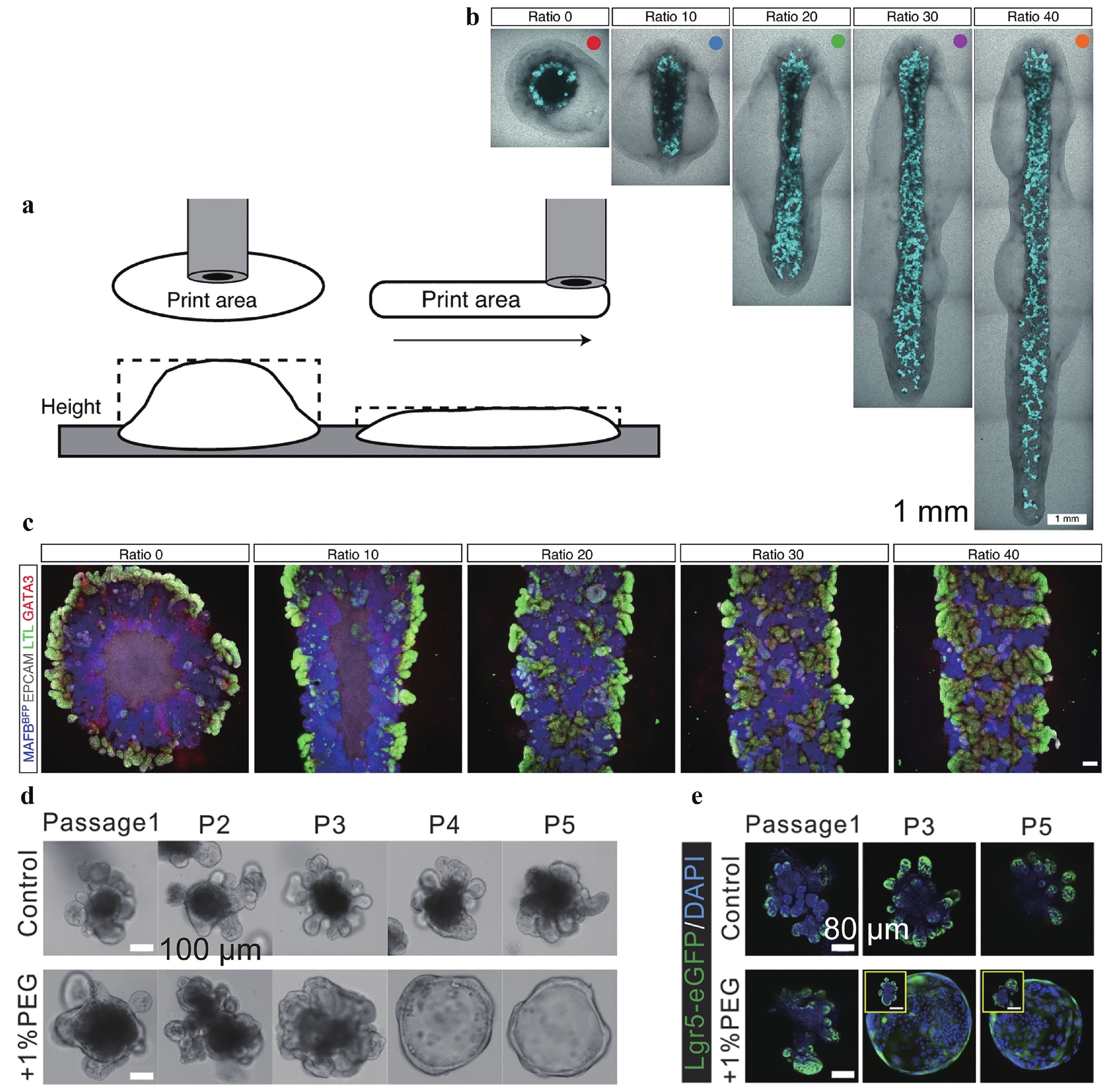
Fig. 2 Scaffold geometry and stiffness regulate stem cell differentiation.
a Sketch of controlled geometry manufacturing. b Image of manufactured microtissues of varied aspect ratios. c Immunofluorescence of representative bioprinted organoids from each conformation showing varied differentiation. d Stiffer scaffold compression expands intestinal organoids and the ratio of intestinal stem cells, indicated by Lgr5-eGFP. Figures adapted from Refs. 21,22 with permission.
-
Cells sense the extracellular environment in many ways, including integrin- and syndecan-based cell adhesion, conformational changes in mechanosensitive proteins (e.g. talin, vinculin, or lamin), activation of mechanosensitive ion channels (e.g. Piezo1), localisation of mechanosensitive proteins (e.g. YAP/TAZ), and downstream activation of transcriptional factors. The cell-matrix interactions crucial for cell fate regulation and proliferation can be selectively reconstructed on synthetic scaffolds by incorporating cell-adhesion motifs. Fibronectin-derived three-amino-acid peptide Arg-Gly-Asp (RGD) is one of the most ubiquitously used peptides to promote cell adhesion to synthetic scaffolds. It binds to αvβ3 and αvβ5 integrins. The cyclic form of RGD, cyclo (Arg-Gly-Asp-d-Phe-Lys) (cRGDfK), was identified as an effective peptide for iPSC culture and performed favourably compared with other peptides derived from laminin, fibronectin, and vitronectin25. Lambshead et al. reported that iPSCs could be maintained for ten passages on cRGDfK brush-coated poly (acrylamide-co-propargyl acrylamide) (PAPA) substrate26.
Synthetic scaffolds have also been used to mimic the role of heparin sulfate proteoglycans, such as perlecan, to support iPSC culture27–31. Klim et al. conducted surface display experiments to identify the ability of different peptides to sustain cell adhesion, including growth factor receptor-binding peptides, integrin-binding peptides, and heparin-binding peptides derived from various structural proteins. The results showed that the heparin-binding peptide GKKQRFRHRNRKG derived from vitronectin supported cell adhesion at the lowest peptide substitution levels. Furue et al. developed a defined serum-free medium for ESC maintenance and proliferation on a type of I-collagen substrate in the presence of heparin. They found that ESCs proliferated in the absence of exogenous FGF-2 if heparin was also present28. Similarly, Nowak et al. showed that poly(ethylene glycol)-heparin (PEG-HEP) matrices could promote the development of human mammary epithelial cells (MECs) into polarised MEC acini in vitro31. Cell remodelling in the post-processing stage generally increases the tissue strength by 1–2 orders of magnitude32.
Mechanosensitive activation of ion channels and other protein effectors is also considered while designing the extracellular scaffold. Yes-associated protein 1 (YAP) nuclear translocation, regulated by mechanical cues, activates downstream transcriptional pathways. YAP/TAZ is a key mediator of mechanosensitive signalling; YAP/TAZ nuclear-to-cytoplasmic ratio is a marker of YAP/TAZ activity, sigmoidally responding to scaffold stiffness. The HAVDI peptide from N-cadherin reduces the mechanical threshold for YAP/TAZ signalling, thus altering MSC interpretation of substrate stiffness15. However, it is not fully clear how YAP/TAZ signalling is regulated by the complex interplay of microenvironmental factors (e.g. stiffness and degradability) and culture dimensionality.
-
In the fields of tissue engineering and regenerative medicine, cells are distributed with certain precision levels (e.g. a few tens to hundreds of microns) before implantation and integration with native tissues33, 34. Cell self-organization, together with scaffold remodelling, further improves organotypic structure development at single and multi-cellular levels35, 36. Scaffold engineering is generally accompanied by controlled sol-gel transition and is strategized with the intrinsic cross-linking properties of the materials, including the reaction mechanism, rate, temperature, and initiation conditions.
A class of materials undergoes sol-gel transition by varying the temperature during molecular assembly. Gelatin, a widely used proteinaceous material derived from natural resources, is solid at temperatures below 30 °C, but liquid at 37 °C37, 38. Matrigel, also naturally resourced and comprised of protein and proteoglycan mixtures, is liquid at 4 °C but crosslinked at elevated temperatures6. The sol-gel transition is reversible. Gelatin is functionalized with methacrylate groups for further photocrosslinking39, or irreversibly cross-linked by a transglutaminase-accelerated reaction40, to improve its thermostability.
Other chemical crosslinking mechanisms have also been employed in the engineering of tissue scaffolds. For example, glutaraldehyde41 and genipin42 can rapidly crosslink proteinaceous materials and their derivatives. However, due to their cytotoxicity, these reactions are mostly used in acellular scaffold engineering, followed by cell seeding, flow-induced cell infiltration, or directed cell migration43, 44. Click reactions offer mild and cytocompatible crosslinking choices, although the reacting components are mostly synthetic or require chemical functionalization45. Divalent ion-induced crosslinking is a class of materials that form chelation nodes between the scaffolding molecular chains and ions, such as Ca2+ and Ba2+ (Refs. 46, 47). Crosslinking is rapid and reversible by extracting ions from a scaffold with a stronger ion-chelating agent.
-
The concept of 4D printing has attracted considerable interest since it was introduced by Skylar Tibbits in 201348. 4D printing emphasises post-printing transformation over time or its shape-changing ability49. The stimuli could be either internal molecules secreted by cells or external signals given by researchers, such as light, heat, ligand, and/or pH shifting. The scaffold needs to be ‘plastic’ and deformed at certain levels during cell culture, contributing to the reconstruction of the extracellular niche and directing the cellular processes. Reconstituted ECM materials used for cell culture, including type-1 collagen gels, Matrigel, and fibrin gels, are typically viscoplastic, unless they are sufficiently crosslinked. In contrast, non-degradable polymer networks would build up stress during cell growth and proliferation and alter cell spreading and migration.
Light is a common stimulus used in laboratories and can be administered remotely. Light initiates strengthened crosslinking, which increases the scaffold modulus or triggers the cleavage of covalent bonds to reduce scaffold stiffness. Stem cell differentiation lineage can be altered via in situ mechanical tunability, and the level of alteration, or plasticity, is negatively correlated with the time point of interference on the scaffold mechanics50, 51. Other post-fabrication stimuli include ligand density and ligating abilities. Cells can modify their niche by secreting enzymes, such as matrix metalloproteinases (MMPs). Consequently, MMP-cleavable peptide modification is a technique widely used in scaffold design. MMP-sensitive gels improve ESC proliferation52. Local administration of MMP inhibitors can attenuate scaffolding degradability and cell remodelling in myocardial infarction53.
-
Filling microstructure moulds with liquid materials produces shaped scaffolds with microscale precision after material crosslinking54, 55. Both solid and liquid shapes (e.g. drops) can be used to engineer moulds. The shapes of liquid moulds are defined by volume, surface tension, and external forces, including gravitational force, adhesion force with the substrate, and viscous force. However, static moulding has intrinsic limitations, including low production capacity and poor structural recovery of soft materials. Various dynamic moulding approaches have been developed to engineer the required structures.
-
Static moulding to shape microgels requires a simple operation, whereby the fluidic precursor solution is cast into the moulds, in which it undergoes in situ crosslinking55, 56. Static moulding enables slow crosslinking reactions, but the production is limited to simple patterns and does not permit distinctive interfaces between different phases. Moreover, the structural recovery of soft microbeads remains challenging.
Lyophilisation has been introduced in static moulding operations to improve structural integrity and pattern complexity. For example, hyaluronan (HA) scaffolds have been obtained with defined shapes and parallel-oriented microchannels by lyophilizing the casted HA solution in the molds57. The microchannel was oriented along the axis of the mould. However, the production rate is still limited, particularly for tissue engineering with microscale precision. Additionally, lyophilisation is not compatible with cell-laden manipulation.
-
Microfluidics can adopt continuous and discrete flow methods, that is, droplet-based microfluidics60, 61. This depends on the nature of the microflows and their wettability with the microchannels. Both microchannels and droplets can be employed as structural moulds to generate fibres and various microbeads.
Monodisperse microbeads are produced using microfluidics droplets as structural templates for crosslinking60. The shapes are dominated by the droplet surface tension when crosslinking occurs under static conditions. At the microscale, the gravitational force is negligible. Surface tension determines that mono-phasic droplets remain spherical throughout crosslinking, thus generating microspheres62. When droplets are multiphasic, the spherical shape is compromised by the joint surface tension among the multiple discrete phases and the continuous phase63, 64. Moreover, the multi-phases within individual droplets can be selectively crosslinked, generating microbeads that adopt concave, convex, or even sharps61, 65.
Microfluidic droplets are usually enclosed with surfactant molecules on interfaces to maintain their stability before crosslinking66. For tissue culturing, it is necessary that the microbeads are removed from the surfactant molecules through repeated washing. When the materials are extremely fragile under shear conditions, such as collagen and Matrigel beads, surfactant removal may render their structural recovery challenging.
Channel confinement allows the production of non-spherical microbeads, such as pancakes and rods. Rapid crosslinking materials can be engineered in short regular channels (e.g. a few centimetres long). Most soft and ultra-soft materials possess slow crosslinking properties, thereby requiring metre-long channels for crosslinking. Meanwhile, minimal disturbance is required during the transition and transport of droplets and beads. Therefore, this requirement is fulfilled with a single long tubing or a cascade of tubing connections with nearly zero dead volumes at the connection ports. Moreover, when channel confinement is effective, the droplet (or bead) occupies the entire interior cross-section of the tubing (channel), thus preventing the bypass of the continuous phase (usually oil). It ensures stable separation and avoids droplet-droplet (and bead-bead) collisions, thus eliminating surfactant-dependent droplet stabilisation, and suits the production of extremely fragile structures38, 58, 59. In addition, the viscosity ratio across the droplet-oil interface can regulate the molecular mixing in droplets, thus offering a route to control the reaction kinetics in a non-chemical way58 (Fig. 3a–3c).
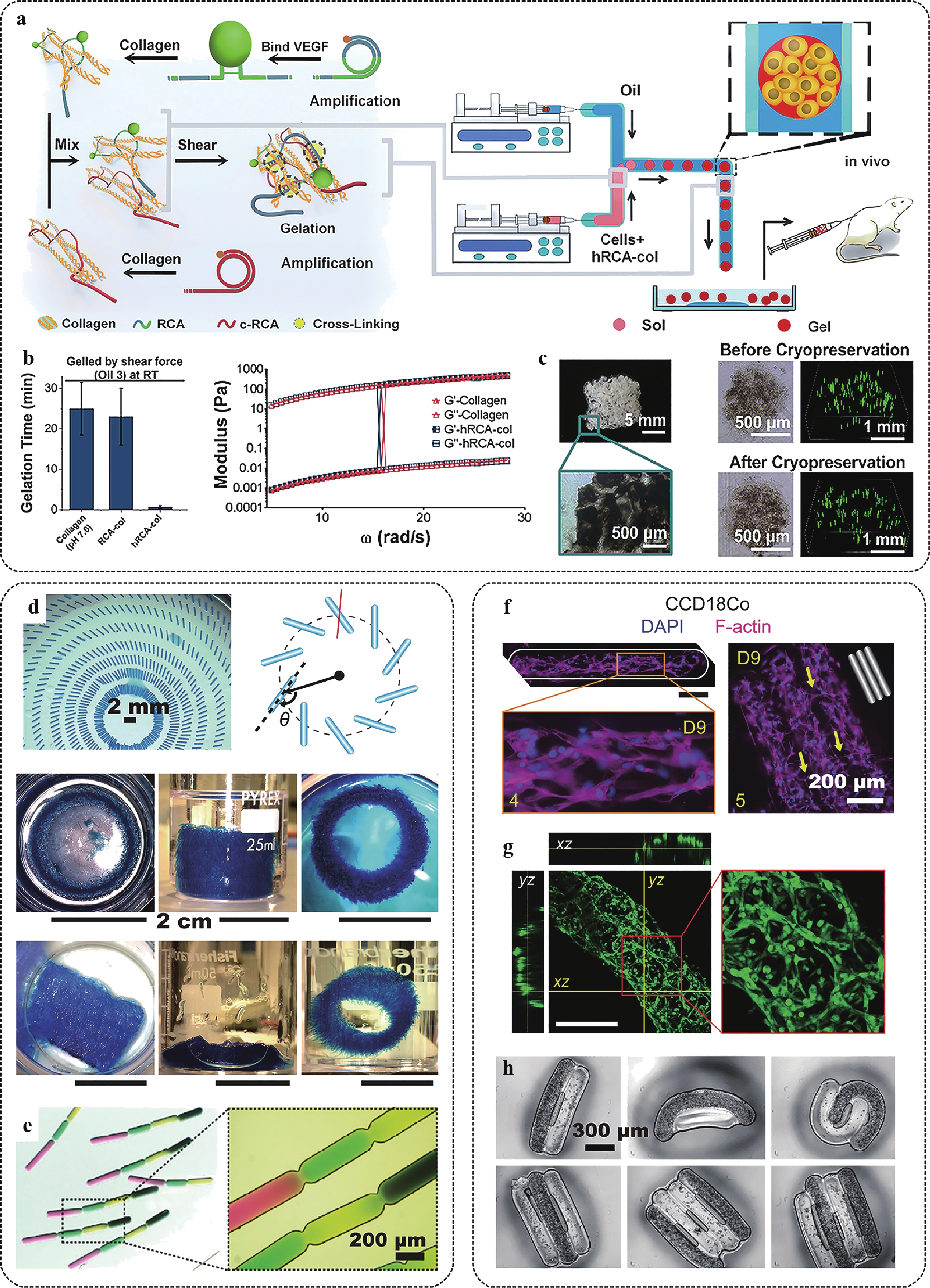
Fig. 3 Manufacturing of ultra-soft microtissues using cascade tubing microfluidics (CTM).
a Sketch of accelerated manufacturing of ultra-soft collagen gel beads in CTM by adopting the ultra-long single strand RCA-DNA interlocking under shear. b Comparison of collagen bead gelation time and shear moduli with (hRCA-col) and without (collagen and RCA-col) the ultra-long RCA-DNA interlocking effect. c Images of moulded hRCA-col microbeads and their cryopreservation. Green fluorescence indicates live cells. d 2D and 3D patterning of gelatinous microrods manufactured in CTM. e Uniform sequences of color-coded microrods. f Morphological development of CCD18Co myoblasts in photocrosslinked gelatin methacrylate. g Morphological development of NIH-3T3 fibroblasts in a Matrigel rod. Fibroblasts formed luminal organization. h Patterned cell laden Matrigel rods driven by hydrophobic effects. Figures adapted from Refs. 38,58,59 with permission.High hydraulic resistance accompanies in-channel crosslinking in long tubing, as the resistance is proportional to the length of the sol-gel transition. To overcome high resistance, fluids should be pumped using high pressure, which may result in high stresses that are detrimental to cells and cause system failure. When cells are encapsulated in droplets for microtissue engineering, mild stress conditions are preferred. Hydraulic resistance can be reduced by employing low-viscosity oil, which generates low friction with the channel walls67. In addition, low-viscosity oil as the continuous phase contributes to milder flow conditions, that is, lower magnitudes of shear and stress levels, in droplets68, 69.
In cascade tubing microfluidics, various microtissues adopting spherical, plugged, and segmented organisations can be produced using mammalian cell-favoured materials, such as collagen, Matrigel, and fibronectin, in their native states6, 70, 71. It promotes cell proliferation, differentiation, and self-organization, thus offering optimised access to uniform organoids, tumoroids, and their controlled assembly36, 72. As many human organs are ordered assemblies of microscale structural and functional repeating units34, such engineered microtissues can be cultured in vitro to function as development and disease models, or as building blocks that compose bioprinting inks or tissue complexes58 (Fig. 3d–3h).
-
Using microchannels as dynamic moulds, moving fluids can be shaped into microfibers via in situ crosslinking in the absence of an immiscible fluid73–75. The hydraulic resistance for a flowing fluid is proportional to its friction area with the channel wall and its viscosity, which is a major issue to overcome in system design. To ensure a continuous flow, the friction area must increase with the length of the in-channel flow path, and its viscosity must increase with the crosslinking process. Therefore, microfibre production via in situ crosslinking is limited to either rapid crosslinking reactions, where the crosslinking path is dramatically shortened73, 74, or addressed via a cascade pumping mechanism, where the resistance is decomposed and overcome in a stepwise pumping manner76 (Fig. 4a–4c). To shorten the crosslinking path, proteinaceous materials, such as collagen, are mixed with alginate that rapidly crosslinks by chelating with Ca2+, providing mechanical stability for collagen before they are fully shaped77, 78 (Fig. 4a, 4b). The channel geometry also contributes to patterning the internal fluid compositions, as the graphene plates are laminated in shallow channel confinement (Fig. 4d).
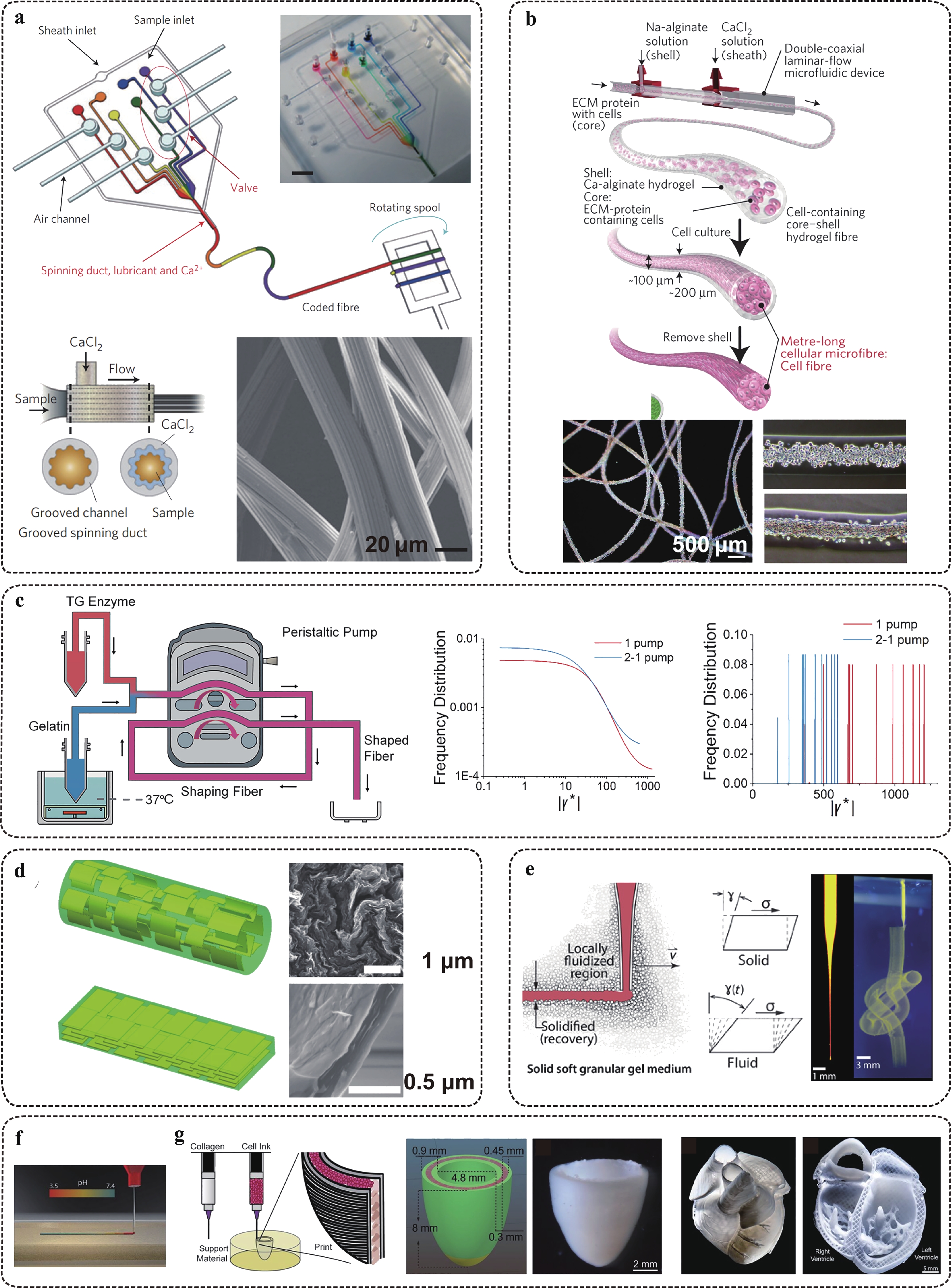
Fig. 4 Manufacturing of soft and ultra-soft tissue fibres with microscale precision.
a Sketch and images of digital microfluidic fibre synthesizer with coded sequence or surface grooves. b Sketch and images of core-shell structured collagen-based tissue fibres. c Reducing hydraulic resistance in in-channel fibre gelation by using cascade pumping. The shear levels are significantly reduced by adopting the 2-pumping scheme. d Microfluidics-based synthesizer of graphene fibres with defined orientation, modulated by flow topology. e Manufacturing of soft fibre-based architecture by using a granular medium (Bingham fluid) support. f, g Manufacturing of a functional heart model by patterning collagen fibre with in situ crosslinking within Bingham fluid buffer f. Figures adapted from Refs. 73–76,79,80 withpermission.Collagen promotes cell self-organization within the porous fibrous scaffold, but alginate lacks the proper cell ligation and degradation abilities. Thus, a homogeneous mixture of collagen and alginate in fibrous shapes limits cell movement. Alternatively, collagen is shaped into a fibrous scaffold in combination with alginate, but adopts the core (collagen)-shell (alginate) separation in their cross-section to achieve synergism between the structural reinforcement and the cell remodelling effect73. This allows cell movement along the core and strengthens the fibre modulus of the shell. Culturing cell-laden scaffolds on a chip is an alternative method to obtain a cell-remodelling environment with supreme structural stability81.
-
Electrospinning82, 83 is an established technique for manufacturing high-strength fibres spanning a few tens to hundreds of nanometres in width. In the electrospinning process, the jet device is filled with a polymer solution or molten liquid. Positive and negative charges are applied separately to the jet device and the receiver. The polymer droplets at the nozzle are filled with electric charges and receive electric force. When the electric intensity increases to a critical value, the electric force overcomes the surface tension. The droplet is ejected from the nozzle and is stretched to the receiver. In this process, the solvent is rapidly volatilized, and the polymers become nanometre-wide fibres. Electrospinning has a high production rate. However, this process is not cytocompatible because of the use of organic solvents and/or high temperatures.
-
3D Printing technology can produce scaffold structures with customised designs, such as beads, rods, fibres, and complex networks in one system84, 85. Microextrusion is widely used in laboratories and has started to prove its potential for industrial use. For soft materials, such as gelatin and its derivatives, the extruded volume can be shaped on the substrate, with a certain level of structure and resolution maintenance40. For ultra-soft and slow-gelling materials, such as collagen and fibronectin, the shaping is supported by relatively rigid and fast gelling materials extruded in parallel and within close proximity86. This effect can also be achieved by using a microparticle gel slurry as the Bingham fluid support, which behaves as a liquid under shear deformation but recovers to solid-state under free-of-shear conditions79 (Fig. 4e). The ultra-soft and fragile liquid is embedded and fixed in the slurry after being extruded. The production of tissue microspheres, fibres, and assembled networks have been proven in the presence of cells.
Alternatively, cells and fibrous scaffolds can be printed separately when the cell suspension is filled into the designed gaps of the scaffold in a layer-by-layer additive process. For example, Lee et al. studied the 3D co-printing of collagen and C2C12 cells80. Two extruders, one filled with the supporting material (collagen) and the other filled with a high-concentration cell ink, were used in the co-printing process. When the acidified collagen solution was extruded into the neutral buffer, it was rapidly neutralised, initiated its gelation, and assembled into a construct under tri-axis guidance (Fig. 4f, 4g).
-
Photocrosslinkable materials, either synthetic or chemically derived natural materials, can be solidified by light radiation in the presence of a photoinitiator. Most initiators are UV light sensitive. Photolithography refers to a scenario in which a designed structure is shaped when the light radiation is patterned89. A photolithography system consists of a light source, a container with the substrate and the prepolymer solution, a series of lenses, and a digital micro-mirror device (DMD) or a physical mask projecting designed patterns87, 90, 91. When the radiation pattern is 3D or illuminated via layer-by-layer patterns in a temporal sequence, a 3D structure with defined variation along the z-axis is obtained92, 93. When photocrosslinking occurs on moving fluids, continuous and reproducible production of coded microbeads is achieved (Fig. 5).
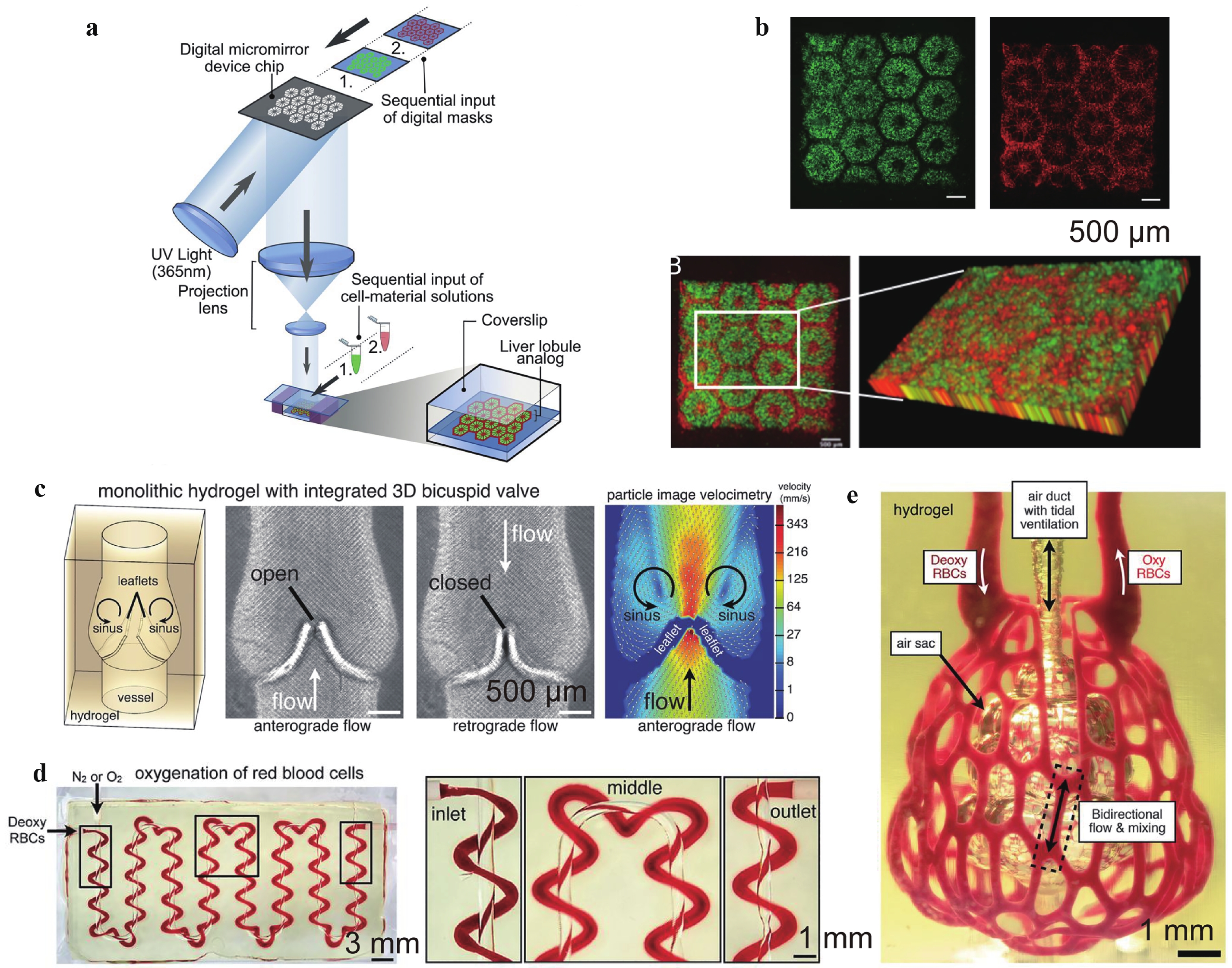
Fig. 5 Photolithography-based additive manufacturing of functional tissues and organ models.
a Schematic diagram of a two-step 3D bioprinting approach in which hiPSC-HPCs were patterned by the first digital mask followed by the patterning of supporting cells using a second digital mask. b 3D reconstruction of the construct showing the patterns of hepatic cells (green) and supporting cells (red). c Photolithography-patterned scaffolds with a functional 3D bicuspid valve integrated into the vessel wall under anterograde and retrograde flows. d Tessellation of the axial vessel and its encompassing helix along a serpentine pathway. Both passages are perfusable and allow the exchange of oxygen. e Photograph of a manufactured scaffold architecture containing the distal lung subunit during red blood cell (RBC) perfusion while the air sac was ventilated with oxygen. Figures adapted from Refs. 87,88 with permission.Photolithography is a technique that is supposed to achieve accurate patterning of cell seeding instead of a random distribution. Its early development focused on patterning adhesive proteins on substrates, and cells were introduced via traditional cell-seeding methods after scaffold fabrication94. However, recent studies have focused on incorporating cells within the scaffold before crosslinking90, thereby improving the cell distribution accuracy, promoting the interaction of cells and scaffolds, and increasing the number of seeded cells. For example, Ma et al. patterned two types of cells using two-step 3D photolithography to mimic the native architecture and cell composition in hepatic tissue87.
Photolithography has advantages over other dimensions. It controls fabrication via external optical signals without invading and disturbing the cell-scaffold interactive environment. It can also conduct scale-up fabrication by enlarging the patterned radiation area, which increases productivity compared to the voxel-based printing techniques95, including microextrusion-based and inkjet printing. However, in terms of structural complexity, voxel-based printing retains its competence in engineering tissues composed of multiple phases and cell types.
Static moulding of soft microbeads
Dynamic moulding of microbeads in microfluidics
Dynamic moulding of microfibers in microfluidics
Dynamic moulding of microfibres using electrospinning
3D printing for microtissue engineering with customization
Photolithography for microtissue engineering with customization
-
It has been acknowledged that most extracellular matrix (ECM) materials are viscoelastic13, although their properties vary in different tissues. To promote cell-matrix mutual remodelling, the materials used are required to possess proper cell ligation and degradation abilities, as well as appropriate porosity. Naturally derived materials include Matrigel (or basement membrane extracts) and decellularized ECM from animal organs. These materials have superior properties to support cell growth, but due to the inconsistency of their resources, these materials suffer lot-to-lot variance, poor reproducibility, high cost, and may cause immunoreactions after transplantation.
Both elastic and viscous materials are easy to synthesise and customise, but viscoelastic materials analogous to native materials are difficult to produce. Additionally, the replacement materials should replicate the properties of porosity, degradability, and communication with cells, to the extent that they are comparable to natural materials. Its scalable production is critical for both basic research and translational applications. The combination of synthetic polymers in conjugation with short peptides is promising, particularly in ultra-large molecular organisations.
-
Although we summarised the methods capable of engineering soft microtissues, their performance with regard to engineering viscoelastic materials is limited in many aspects. For example, photolithography is limited to photocrosslinkable materials, and flow lithography requires the microstructures to be persistent against flow-induced shear deformation; microextrusion suffers from resolution loss resulting from slow crosslinking in the absence of channel confinement, or friction-induced structural loss when crosslinked in-channel. In the removal of surfactants using microfluidic droplets as structural templates, shear forces during washing and phase transfer would result in the collision and collapse of the fragile microbeads. Cascade tubing microfluidics (CTM) in the absence of surfactants eliminates the need for repeated washing and eases phase transfer, but the use of oil to disperse the cell suspension droplets may result in oil residues and also introduces extra steps, thereby increasing the cost of operation. Novel engineering methods for engineering ultra-soft and viscoelastic materials are in high demand.
-
Lastly, the capacity for live tissue engineering with microscale precision always faces a conflict between laboratory and industrial needs. Both microfluidics- and 3D bioprinting-based engineering techniques exhibit superior performance as state of the art demonstrations. Owing to their intrinsic limitations, a single module set has a limited production ability. Numbering up the running modules can scale-up production, but the cost and high-parallel control emerge as new issues. Meanwhile, the miniaturisation of the control modules in the aforementioned techniques is a common challenge. The 3D printed tissues have larger scales but can be regarded as scaled-up examples of microtissues in an ordered assembly. Further scaling-up of the printing modules might pave the way toward industrial manufacturing of soft and ultra-soft tissues.
In conclusion, engineering soft and ultra-soft scaffolds in a cytocompatible and yield-friendly manner has fundamental impacts on both basic and translational medicine. Techniques such as these are expected to offer breakthroughs and accelerate disease studies, drug discoveries, personalised medicine, and regenerative therapies. Its interdisciplinary nature requires joint inputs from materials, biology, chemistry, medicine, and mechanical and electronic engineering to overcome the modular challenges.
Lack of viscoelastic materials that recapitulate the same mechanical properties as the native scaffolds
Engineering methods for viscoelastic soft materials
Production conflict in the laboratory and scale-up
-
This work was supported by the National Natural Science Foundation of China (Grant Number: 61971255), Shenzhen Bay Laboratory Fund (Grant Number: SZBL2020090501014), and Shenzhen Science and Technology Innovation Committee (Grant Number: KCXFZ202002011010508).






 DownLoad:
DownLoad: