-
Carbon dots (CDs) have emerged as novel fluorescent nanomaterials with excellent tunable fluorescence characteristics, electron donor and acceptor abilities1, biocompatibility, low toxicity, and solution processability2–6. These advantages endow CDs with immense potential in various fields, including optoelectronic devices, sensing, imaging, detection, and fluorescence anti-counterfeiting7–10. CDs generally comprise sp2 or sp3 hybridized carbon nuclei with rich functional groups or polymer chains on their surfaces11. The functional groups surrounding the carbon core and its edges, and the molecular fragments with special functions, grant CDs an extremely high structural complexity and tunability. Since the discovery of CDs, various synthesis methods have been developed to improve their optical properties, including arc discharge, laser ablation, electrochemical oxidation, solvent thermal carbonization, template carbonization, and microwave irradiation12,13. However, a single CD synthesized using a single precursor without doping with heteroelements such as nitrogen, boron, phosphorus, and sulfur in the carbon skeleton has inherent drawbacks, including susceptibility to fluorescence quenching in aggregated states and a low quantum yield (QY). Traditional CDs have limited practical application owing to their low QY. Therefore, an increasing number of studies have focused on exploring different strategies to optimize the fluorescence characteristics and performance of CDs. Fine-tuning the chemical structure of the carbon cores and surfaces is necessary for the pursuit of improved properties. The two primary methods used to expand the applicability of CDs are surface functionalization and chemical doping. Surface functionalization (i.e., regulating the functional groups on the surfaces of CDs) can generate or alter the surface states of CDs and provide them with various functions through binding with functional molecules or polymers. The doping of heterogeneous elements into the carbon framework of CDs using multiple precursor materials is called chemical doping14. Among the various optimization strategies, doping is regarded as one of the most effective methods for controlling the electrical, optical, and chemical properties of CDs. In general, compared to undoped CDs, doped CDs (X-CDs, where X represents the doping element) possess the advantages of longer fluorescence lifetimes, higher QYs, and better fluorescence intensities. Various atomic doping impurities can be introduced to regulate the structure and electron distributions of CDs, such as nitrogen15,16, boron17, sulfur18, and phosphorus19, or metal ions such as Na+, Fe3+, and Zn2+. With metal or non-metal doping, the generation of n- or p-type charge carriers and changes in the bandgap energy can improve the photoluminescent performance of CDs.
Numerous reviews20–22 have focused on X-CDs, most of which concern doping with non-metallic heteroatoms, such as nitrogen, sulfur, boron, or halogens. There have also been several studies on metal-ion-doped CDs23,24, such as Cu2+, Mg2+, and Mn2+. However, systematic reviews on the mechanisms and application of both metal and non-metal doping and their effect on the optoelectronic properties of CDs are currently lacking. Therefore, in this article, we discuss and highlight the effects of various types of CD doping by introducing the recent progress in metal and non-metal X-CDs. Moreover, we summarize the advanced applications of X-CDs, including optoelectronic devices, information encryption, anti-counterfeiting, imaging, and detection. The prospects and challenges of X-CDs, which are expected to provide ideas for further research, are also discussed.
-
The introduction of heteroatoms affects the overall charge distributions and electronic energy levels of CDs, and doping has proven to be an effective method for regulating the fluorescence and other properties of CDs. To date, CDs have been doped with various heteroatoms (nitrogen, sulfur, boron, selenium, tellurium, phosphorus, silicon, and halogens). The specific doping atoms can change the electrical structure of CDs, suppress their surface defects25, and improve their performance. On one hand, X-CDs can be divided into mono-doping or co-doping CDs according to the number of doping heteroatoms. Unlike single-atom doping, co-doping utilizes the synergistic effect between multiple heteroatoms to promote the formation of special electronic structures in the molecules, thereby improving the fluorescence characteristics of CDs11. On the other hand, heteroatom doping includes both non-metal and metal doping, according to the introduced heteroatom. Because the sizes of the non-metal atoms are similar to that of carbon, the resulting effective and uniform doping enables the simple synthesis of such X-CDs. Research on the non-metal doping of CDs has mainly focused on nitrogen, sulfur, and boron atoms, as well as halogens and other elements. Metal ions have more electron and unoccupied orbitals, which can effectively change the energy gaps and electron density distributions of the CDs, thereby altering their optoelectronic properties.
-
Mono-atom doping with nitrogen, a frequently utilized element in CD doping, has been extensively employed to enhance the optical properties of CDs. Because nitrogen atoms have five valence electrons and an atomic radius similar to that of carbon, it can form covalent bonds with the carbon atoms, thereby changing the photoelectric properties of the CDs. Research has shown that nitrogen doping can regulate the gap between the lowest unoccupied molecular orbital (LUMO) and the highest occupied molecular orbital (HOMO) of CDs, thereby affecting their photoluminescence (PL). Lu et al26. found that, after doping with nitrogen, the interaction between graphitic nitrogen and C=O extended the effective conjugation length, thereby reducing the energy gap between the HOMO and LUMO and facilitating the enhanced fluorescence of the N-CDs. Compared to undoped CDs, nitrogen doping alters the surface state of N-CDs, generating new energy levels and resulting in a significant red shift in the fluorescence emission wavelength. Recently, Liu et al27. compared the LUMO and HOMO of N-CDs and found that when sufficient energy was provided, electrons in the HOMO could be excited into the LUMO, and the band gap energy decreased from 2.01 to 0.64 eV. This enhanced the fluorescence of the N-CDs. Their study showed that the nitrogen doping not only causes a redshift in the CD emission peak but also enhances the fluorescence owing to the fewer chemical states and increased number of surface defects on the CD. In addition, doping can induce the formation of more sp2 hybridization sites, thus enhancing the fluorescence. The absorption intensity is also enhanced owing to the interaction between the unpaired electrons of nitrogen and the functional groups of the CDs. Ye et al28. prepared CDs with excitation-dependent fluorescence, as shown in Fig. 2a, using 5-amino-phenylenedicarboxylic acid as the precursor and ammonia, ethylenediamine, and hydrazine hydrate as three additional nitrogen sources (Fig. 2b). As shown in Fig. 2c, the LUMO–HOMO gap decreased by more than 3 eV, and the CDs exhibited stronger fluorescence emissions at long wavelengths. Furthermore, studies have shown that nitrogen doping can increase the number of defects and surface states of CDs, which is beneficial for capturing more electrons and increasing the probability of radiative recombination, thereby enhancing the fluorescence. In N-CDs29, the existence of C=N bonds enhances the conjugated structure, resulting in more defects and an enhanced PL.
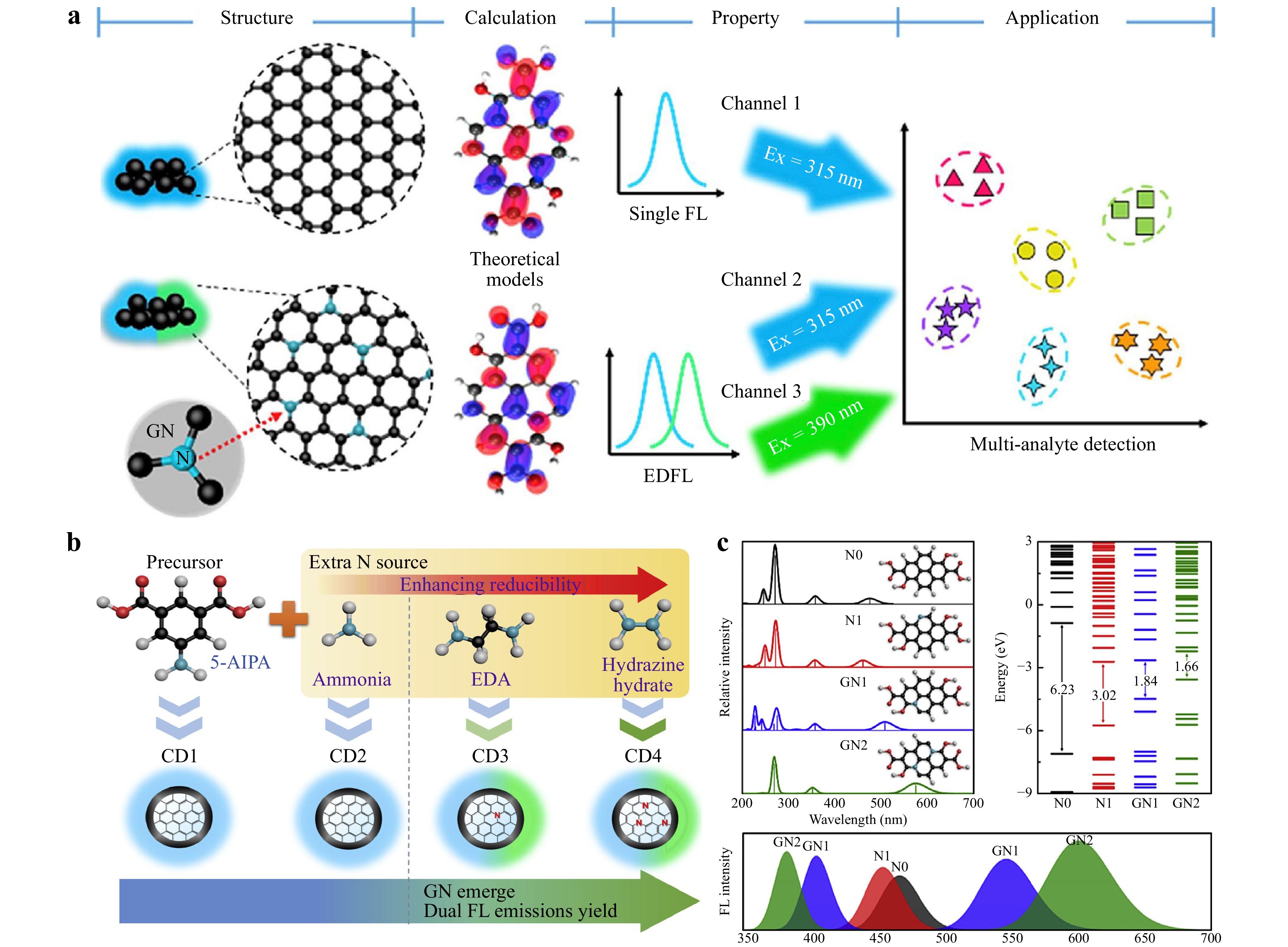
Fig. 2 a Structure and properties of carbon dots. b Synthesis of carbon dots. c Energy bands of various carbon dots. Reproduced with permission28 (Elsevier 2021, Dyes and Pigments).
-
In addition to nitrogen, sulfur is also widely used to dope CDs to improve their PL and endow them with unique properties. Nitrogen and sulfur atoms introduced into CDs will produce n-type charge carriers. In contrast to boron, nitrogen, and phosphorus, the radius of the sulfur atoms is larger than that of carbon, and the length of the C-S bond is 25% longer than that of the C-C bond. Because of the similar electronegativities of sulfur (2.58) and carbon (2.55), S-doping has less of an effect on modulating the charge transfer of the CDs. Although there are difficulties in doping sulfur into the molecular chains of CDs30, it can still form covalent bonds with carbon atoms, thereby affecting the geometry and electronic structure of the CDs31. Doping with electron-rich sulfur causes the HOMO energy level of the CD to shift upward, while only slightly changing the LUMO energy level. The resulting decreased energy band is beneficial for improving the PL performance of CDs. Mombru et al32. investigated the optoelectronic properties of CDs doped with sulfur and nitrogen atoms at the center and edge. They discovered that sulfur doping is more difficult than nitrogen doping, and that edge doping results in a better performance than center doping. In terms of the optical properties, S-CD transitions are located at wavelengths lower than those of undoped CDs. Wang et al33. reported new S-CDs with preferable fluorescence performances, which were prepared via hydrothermal synthesis using Na2S as the sulfur source and citrate as the carbon precursor (Fig. 3a). Owing to the fewer surface defects of the S-CDs, which reduced the probability of non-radiative electron transitions, the fluorescence intensity of the S-CDs was higher than that of the undoped CDs (Fig. 3b). More electrons radiated in the form of photons, thereby increasing the fluorescence intensity. Moreover, the effect of the sulfur doping ratio on the fluorescence intensity of S-CDs is complicated. In the case of low sulfur doping, most sulfur atoms participate in the deprotonation process to produce H2S, rather than directly participating in the nucleation of S-CDs. This non-nucleation-participation leads to a reduction in the fluorescence intensity. However, when the sulfur doping ratio increases to a higher level, the repulsion between sulfur atoms is significantly enhanced, preventing the effective formation of S-CDs. This also leads to a decrease in fluorescence intensity. These two mechanisms both negatively affect the fluorescence intensity of S-CDs.
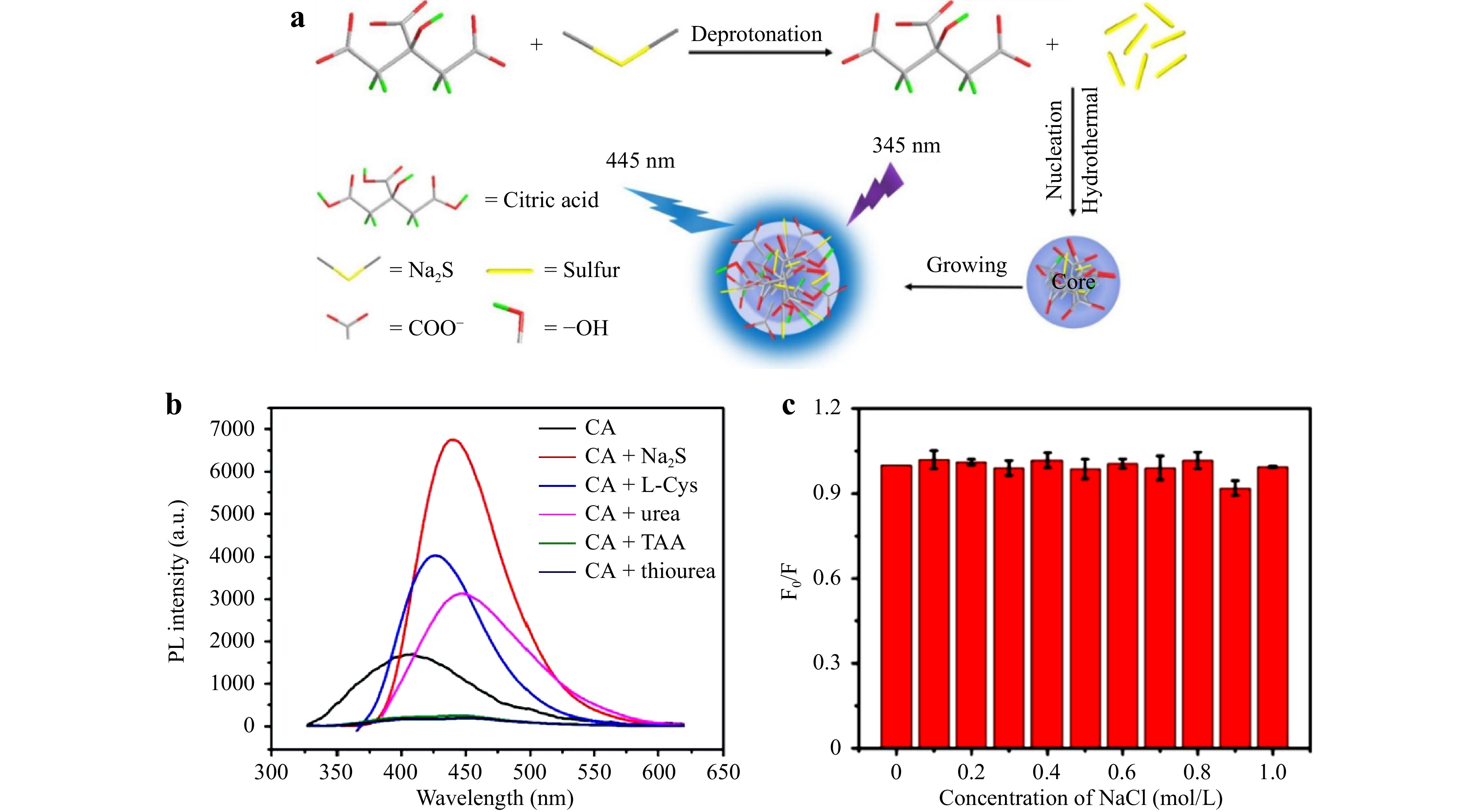
Fig. 3 a Schematic of the synthesis of sulfur-doped carbon dots. b Comparison of fluorescence intensities with different doping sources. c Fluorescence stability of carbon dots. Reproduced with permission33 (Elsevier 2019, Materials Chemistry and Physics).
-
On the periodic table, boron is the left-side neighbor of carbon, and their atomic radii are similar. These two elements share similarities in structure and physicochemical properties and can form B-C covalent bonds, suppress CD defects, and modify CD optoelectronic properties34. Nitrogen (5) and sulfur (6) have more valence electrons than carbon (4), whereas boron has only three valence electrons. Therefore, if boron atoms are introduced into CDs, the internal structure of the CDs produces p-type charge carriers. The significant difference in electronegativity between carbon (2.55) and boron (2.04) can cause considerable charge transfer in B-CDs30. However, in contrast to the HOMO energy level shift caused by doping with electron-rich nitrogen and sulfur, in B-CDs the LUMO energy level shifts downwards and the HOMO energy level change is relatively small. In addition, the length of the B-C bond is 0.5% longer than that of the C-C bond, and the electronic defects of boron promote the generation of defects in the energy state of B-CDs, leading to the emission of defects on their surfaces35. Taymaz et al36. prepared B-CDs using boric acid as a boron source. They found that the interaction between boron and the CDs led to a decrease in the bandgap energy, allowing more light to be obtained at long wavelengths. Compared to undoped CDs, B-CDs have a higher PL strength, which may be related to the CD defects. Current research on the effect of boron doping on the fluorescence of CDs mainly focuses on improving the QY or fluorescence intensity, promoting the redshift of the emission wavelength, and multiple emissions22. Zhao et al37. synthesized B-CDs using a solvothermal reaction. The combination of boron and carbon disturbs the structure of a thiophene ring, and the carbon configuration in the CDs is altered by the boron doping, resulting in a surface defect state. Therefore, compared to undoped CD, B-CDs have a higher fluorescence and a redshift of approximately 38 nm.
-
Phosphorus is a member of the nitrogen group of elements and has demonstrated doping effects analogous to those of nitrogen. Being an electron-rich entity, phosphorus effectively diminishes the HOMO–LUMO band gap by increasing the HOMO energy, culminating in a redshift of the emission spectrum and a notable enhancement of the fluorescence properties of the material. This phenomenon is attributed to the interaction between the phosphorus atoms and the carbon structure during synthesis, which introduces additional electrons—thereby inducing n-type doping—and substantially modifies the electronic structure and surface chemical activity of the material. These alterations have profound implications on the optical properties of the material. Single-atom doping, particularly the integration of phosphorus atoms, greatly modifies the electronic structure and optical properties of CDs, thereby significantly enhancing their PL performance. Generally, with increasing concentrations of phosphorus heteroatoms in CDs, the population of surface and defect states multiplies. These states exhibit a higher capacity for electron trapping, ultimately resulting in enhanced fluorescence QY. Sarkar et al38. synthesized phosphate-doped green fluorescent CDs. The increase in the fluorescence intensity and QY of the P-CDs were attributed to an increase in the number of isolated clusters of sp2 carbon. They hypothesized that the coexistence of defect sites and isolated sp2 carbon clusters effectively increases the band gap in the UV–visible region, and thus produces stronger fluorescence relative to a lone sp2 carbon. By doping phosphorus into CDs, a spectral transition from blue to green was achieved, accompanied by a high QY. Kalaiyarasan et al39. successfully synthesized P-CDs using a hydrothermal approach employing trisodium citrate and phosphoric acid as precursors. Their study revealed that an increase in the concentration of phosphorus dopants in P-CDs effectively passivated the surface states, leading to monochromatic fluorescence. Specifically, the P-CDs exhibited monochromatic emission when the P/O doping ratio exceeded 0.1 (P/O > 0.1), whereas they displayed polychromatic emission at a P/O ratio below 0.1 (P/O < 0.1).
-
Halogen (fluorine, chlorine, bromine, and iodine) doping can also increase the QY and improve the photophysical properties of X-CDs. Fluorine is the chemical element with the highest electronegativity and can absorb adjacent electrons and enhance the separation of positive and negative charges. Fluorine edge doping replaces hydrogen atoms in conjugated organic molecules, forming covalent bonds through sp2 hybridization and maintaining the conjugated π system of the carbon nucleus40. Li et al41. synthesized the F-CD products FCDs-1 and FCDs-2, among which FCDs-1 have a lower fluorine content than FCDs-2. According to calculations, the optical bandgaps of undoped CDs, FCDs-2, and FCDs-1 are 3.49, 3.21, and 2.98 eV, respectively. This results in a redshift in the PL emission of the CD products that increases with increasing fluorine content. This phenomenon indicates that fluorine alters the HOMO and LUMO energy levels of CDs, thereby affecting their PL emissions. Huang et al42. synthesized high-fluorescence CDs doped with fluorine using tetrafluorobenzoquinone (TFBQ) as a fluorine source. Their study found that fluorine doping increased the CD crystallinity. The QY of the F-CDs excited at 360 nm reached 39%, which was more than twice that of the undoped CDs (15%), further proving that fluorine doping can effectively improve the fluorescence efficiency of CDs. Further, the chlorine doping of CDs enhances the separation of photoexcited charge carriers, which has been proven to form additional energy levels. This is beneficial for improving the optoelectronic performance of CDs43. Murali et al44. synthesized typical Cl-CDs terminated by carboxylic functional groups using sucrose and sucralose as raw materials. This chlorine doping strategy improved the photoelectric performance of the CDs by adjusting their photoelectric energy levels. The study found that the band gap of Cl-CDs is 3.24 eV, much narrower than the band gap of unchlorinated X-CDs (4.07 eV). Because of the significant optical band gap narrowing and reduced charge recombination owing to an internal electric field originating from band bending, the Cl-CDs showed highly efficient visible-light-driven photodegradation, opening a new design avenue for X-CDs. In reports on halogen-doped CDs, fluorine and chlorine are primarily used, and few studies have focused on I45 or Br46 doped CDs.
-
The introduction of metal ions can alter the electronic structure, chemical composition, and physicochemical properties of CD nanostructures. Compared to non-metallic elements, metal ions have more unoccupied and electron orbitals, as well as larger atomic radii. Doping with metal ions can effectively change the energy gap and electron density distribution of CDs, thereby altering their optical and electronic properties; however, it can also introduce toxicity22. In recent years, metal-doped CDs have received increasing attention owing to their unique advantages in charge transformation and charge density regulation. Recently, Sun et al47. used nickel chloride (NiCl2) as a metal source and doped CDs with nickel in the form of Ni-N bonds, thereby preparing Ni-CDs. Nickel doping increases the number of photogenerated charges in CDs, enhances charge transfer, and facilitates the radiative recombination of charge carriers. Simultaneously, the Ni-N bonds fill the vacancies in the CDs, effectively suppressing their non radiative transition recombination, and improving their fluorescence QY. The absolute fluorescence QY of Ni-CDs at 350 nm is 54.7%, which is 14% higher than that of undoped CDs. In addition, nickel doping alters the energy-level structures of the CD, thereby prolonging their fluorescence lifetime. Through doping CDs with sodium, Na-O CDs have been reported48. After sodium doping, Na–O functional coordination junctions were formed on the surface of the CDs, introducing new energy states. The Na-O—as the defect luminescent center—causes a decrease in bandwidth and enhances the QY of deep-blue light emission. In addition, sodium doping effectively suppresses non-radiative decay, increases the PL lifetime, and increases the PL QY from 1.45% to 19.36%. Gong et al49. synthesized manganese-doped CDs (Mn-CDs) using manganese acetate. Under UV irradiation at 365 nm, the Mn-CD powder exhibited solid red fluorescence. The study found that doping with manganese ions was an important factor in the solid red fluorescence of the CDs. Xu et al50. prepared zinc-doped CDs (Zn-CDs), with zinc ions acting as passivating agents on the surface of the CDs, effectively preventing the aggregation of π–π stacking in the structure, thereby increasing the QY of Zn-CDs. Metal-ion-doped CDs can effectively change the charge transformation form and electron density between CDs and metal ions by combining them with CDs with high electron mobility, thereby regulating the physical and chemical properties of CDs. This may induce new physical and chemical properties in the CDs, such as catalytic properties24. To date, CDs doped with different metal ions have been prepared; however, research on metal doping is far less extensive than that on non-metals.
-
Single-atom doping has shown significant potential for regulating the intrinsic properties of CDs. However, many studies have also shown that co-doping with two or more different atoms can result in synergistic effects, forming unique internal structures and improving the optical properties of CDs. As such, co-doping technology has also received significant attention.
-
Nitrogen and boron are the atoms adjacent to carbon on the periodic table and have very similar atomic radii. This makes it easy to achieve nitrogen doping as an electron and boron doping as a hole in the CD structure. After doping CDs with nitrogen and boron, the conduction or valence band positions of the CDs change through the synergistic effect of the two atoms, improving the CD conductivity and optical properties. Therefore, the co-doping of nitrogen and boron has received widespread attention in recent years.
Xu et al51. synthesized boron- and nitrogen-doped CDs (BN-CDs) using waste tea residue as the raw material and boric acid as the boron source. The specific synthesis process and reaction mechanism are shown in Fig. 4a. Nitrogen doping can effectively inhibit defect states on the CD surface, thereby improving the PL performance. By doping boron in the form of holes in the CD structure, p-type carriers are generated, and their electronic structure is changed such that the QY excited at 379 nm increases from the initial 11.4% to 25.79% (Fig. 4b). Tran et al52. prepared BN-CDs using passionfruit juice as the raw material and boric acid as the boron source, which has a higher electron affinity than N-CDs. In addition, highly electronegative groups can increase the electron deficiency inside the CDs and increase the QY of BN-CDs compared to that of N-CDs. Shokri et al53. prepared BN-CDs using citric acid, boric acid, and urea as the raw materials. The QY of the doped CDs was higher than that of undoped ones. The nitrogen and boron co-doping greatly improved the electron and hole radiation recombination efficiency of the BN-CDs, leading to a higher QY. Nitrogen–boron co-doping has been widely studied, but there have also been reports on nitrogen–phosphorus and nitrogen–sulfur multi-atom co-doping.
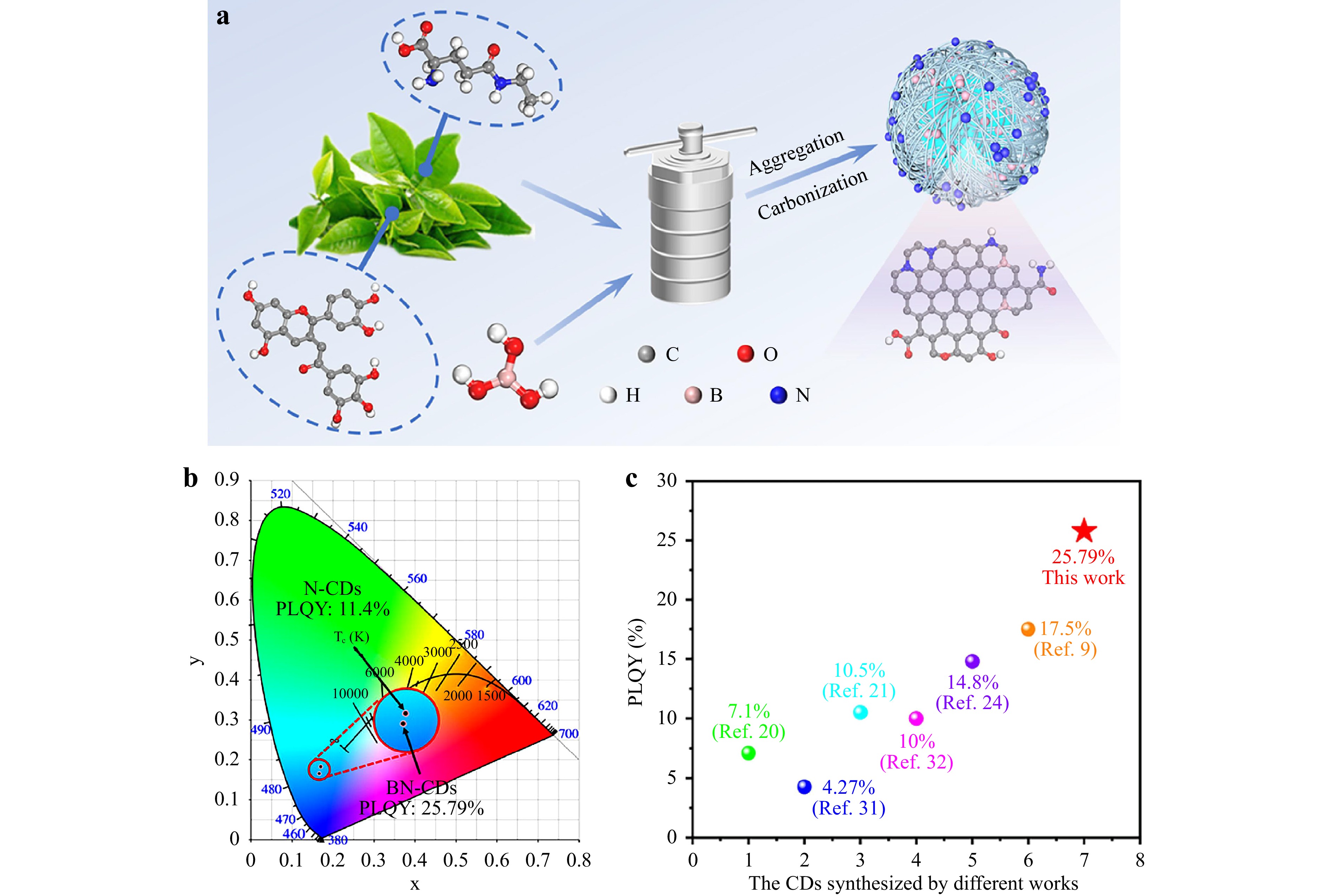
Fig. 4 a Synthesis process and reaction mechanism of boron–nitrogen (BN)-CDs. b CIE chromaticity diagrams of BN-CDs and N-CDs. c Photoluminescence QY of CDs from different studies. Reproduced with permission51 (Elsevier 2023, Ceramics International).
-
After doping with nitrogen, CDs become n-type. Electron-rich phosphorus has a high chemical activity and can change the electronic properties of CDs by co-doping with nitrogen and phosphorus atoms, thereby improving their optical properties. Bao et al54. prepared NP-CDs using a hydrothermal method and showed that the QY of NP-CDs was higher than that of undoped CDs. Because of the strong electron-withdrawing ability of the nitrogen, oxygen, and phosphorus atoms on the surface of the CDs, the active sites on the surface were effectively passivated. This promotes the stabilization of excitons, thereby altering the electronic structure of the CDs and facilitating the radiative recombination of charge carriers. Yashwanth et al55. synthesized NP-CDs using ethylenediamine as the nitrogen source and phosphoric acid as the phosphorus source. Owing to the introduction of nitrogen and phosphorus, the Fermi level shifted from the valence band edge of the single CDs to the conduction band edge, thereby reducing the work function and facilitating the generation of photoelectrons.
-
Nitrogen–sulfur co-doping can effectively regulate the band structure of CDs, narrow their bandgap, and improve their visible-light absorption capacity, thereby improving their optoelectronic performance. Further, the nitrogen–sulfur co-doping can enhance the fluorescence intensity of the CDs and adjust the position and wavelength of their emission peaks56. Wu et al56. prepared NS-CDs using a hydrothermal method. Compared to N-CDs, the introduction of sulfur atoms disrupts the covalent bond between The nitrogen and carbon, reducing the dependence on absorbed light energy, which is independent of excitation. Sulfur doping introduced new energy levels, improved the band structure and fluorescence performance of CDs, enhanced their excitation independence, and increased their PL QY from 11.2% to 65.1%. When nitrogen–sulfur co doping is performed, the band gap is significantly reduced. This results in a corresponding decrease in the HOMO–LUMO gap, thereby promoting a decrease in the optical transition energy. Liang et al57. synthesized NS-CDs using a solvent and thermal synthesis of sodium alginate (SA) and glutathione (GSH) as raw materials. NS-CDs-1, NS-CDs-2, and NS-CDs-3 were synthesized based on SA/GSH mass ratios of 0.48, 0.25 and 0.125, respectively. These NS-CDs exhibited dual emission, excellent photostability, and tunable fluorescence at 480 and 650 nm. Nitrogen and sulfur doping affected the electronic structure of the NS-CDs by regulating the generation of different types of charge carriers, thereby altering their optical properties. Reducing the SA/GSH mass ratio can increase the size of NS-CDs, reduce the nitrogen content, and ultimately enhance the PL, transforming the fluorescence from cyan to white-blue. Doping can form abundant defect sites, which may reduce the probability of electron delocalization around the NS-CDs, leading to multiple emission traps. The three NS-CD aqueous dispersions appeared transparent and light-brown/green under sunlight. When irradiated with a 365-nm ultraviolet lamp, these aqueous dispersions emitted cyan, light blue, and white-blue fluorescence, respectively. By adjusting the SA/GSH mass ratio, the fluorescence-tunable performance of the NS-CDs can be realized.
-
According to the theory of semiconductor physicochemical properties, similar to most co-doping methods, the electronic structure and intrinsic properties of CDs are altered by the properties of different elements, thereby increasing the fluorescence QY. Currently, the co-doping of nitrogen and other atoms includes nitrogen–fluorine co-doping (NF-CDs)58,59, nitrogen–chlorine co-doping (NCl-CDs)60,61, nitrogen–oxygen co-doping (NO-CDs)62, and nitrogen–silicon co-doping (NSi-CDs)63. In addition, there are three-element co-doping methods, such as nitrogen–sulfur–phosphorus co-doping (NSP-CDs)64,65, nitrogen–sulfur–boron co-doping (NSB-CDs)66,67, and nitrogen–phosphorus–boron co-doping (NPB-CDs)47,68. Liu et al.69 reported the synthesis of fluorine–nitrogen co-doped CDs (NF-CDs) using diethylenetriamine and fructose as precursors. They speculated that the introduction of fluorine atoms enhanced the planarity of the molecule and formed hydrogen bonds between the C-F bonds and substituents, thereby increasing the fluorescence intensity. Further, the fluorescence QY increased from 25.80% for the N-CDs to 51.94% for the NF-CDs. Studies have shown that silver is a strong Lewis acid with strong affinity for nitrogen donor atoms. Therefore, stable complexes could be formed between silver and nitrogen through electron transfer, facilitating the synthesis of NAg-CDs. Lakshita et al.70 synthesized N-CDs, NAg-CDs, and nitrogen and cerium co-doped CDs (NCe-CDs) by a hydrothermal method. All three CDs showed chemical inertness, and doping reduced the surface defects. In NCe-CDs, the co-doping of nitrogen and cerium increases the electron density on the surface of the CDs, thereby enhancing their fluorescence intensity. The QY of N-CDs, NCe-CDs, and NAg-CDs have been reported at 26.6%, 29.4%, and 30.2% in the research, respectively. Owing to nitrogen doping, the HOMO–LUMO gap of NAg-CDs is reduced, causing a redshift and enhanced photoluminescence, thereby improving the radiative recombination. Huang et al.71 synthesized NCl-CDs using phenylenediamine and aluminum chloride hexahydrate as raw materials. According to previous studies, the effective doping of chlorine diversifies the electronic transition energy levels and enhances the tunability of the emission wavelengths. The co-doping with chlorine and nitrogen increases the transition of electrons from the occupied energy levels and generates additional energy levels, thereby changing the energy gap. In addition, a high defect density increases the likelihood of electrons being captured by defects and increases the number of emission centers of the NCl-CDs. Therefore, the synergistic effect of these two effects leads to the wide-ranging emission of NCl-CDs.
-
At present, there is relatively little research on the mixed doping of metals and nonmetals. The studies that do exist focus mainly on NFe-CD mixed doping72. The doping of iron ions and the generation of defects in the bandgap increases the carrier concentration, thereby increasing the local electron density and facilitating intermolecular electron transfer73. Nitrogen doping promotes the entry of photogenerated electrons into the conduction band73. Luo et al.74 prepared NFe-CDs using a hydrothermal method, and the resulting NFe-CD solution was brownish yellow in color. Doping with iron ions increases the local electron density, which enhances the catalytic activity for intermolecular electron transfer. Yue et al.75 synthesized NFe-CDs and used them to detect H2O2 and uric acid. Ramos et al.76 synthesized CDs co-doped with nitrogen and lanthanide metals using two lanthanide salt elements as metal dopants, coordinating and combining lanthanide ions with the CDs. The synthesized CDs had good stability, high fluorescence intensity, and a high QY of 66 ± 7%. Meng et al.77 synthesized cobalt–nitrogen-doped CDs using a low-temperature polymerization solvent extraction method. Cobalt exists in the form of Co2+ within the CDs, which facilitates electron transfer within the internal structure. Therefore, co-doping can enhance the optoelectronic properties of CDs. Sun et al.78 prepared novel NFe-CDs by electrolysis; NFe-CDs-2 exhibited very stable fluorescence and retained over 95% of its initial value after being stored in solution for seven days. The formation of nitro, carboxyl, hydroxide, and amino groups on the surface of NFe-CDs-2 improved its stability in aqueous systems. Compared with N-CDs, owing to the iron doping, the electron transition from iron to nitrogen in NFe-CDs-2 caused charge transfer between the metal ligands. Therefore, under the same conditions, the absorption spectrum of NFe-CDs-2 exhibited a stronger absorption band in the range of 260–310 nm. The NFe-CDs were successfully used for the selective detection of Cu2+ ions. Xu et al.79 synthesized non-metallic nitrogen and bimetallic zinc–cobalt co-doped carbon dots (NZnCo-CDs). Nitrogen can be doped into carbon nuclei to generate nitrogen functional groups on the surface, whereas bimetallic doping can protect the functional groups formed on the surface of the CDs, effectively enhancing the sensitivity and fluorescence intensity of NZnCo-CDs as fluorescent probes.
-
Light-emitting diodes (LEDs) have become the primary technology of the modern display industry owing to their advantages of self-illumination, all-solid-state, and high electro-optic energy conversion efficiency. They play an important role in the new generation of flat-panel displays and solid-state lighting. CDs are novel zero-dimensional semiconductor nanoluminescent materials that exhibit excellent tunable fluorescence properties, low toxicity, and solution processability. They are ideal substitutes for traditional luminescent materials in optoelectronic devices and are widely used in the field of luminescent devices. Based on the principle of luminescence, CD-based LEDs can be classified into photoluminescent and electroluminescent devices. Electroluminescent devices directly use CDs as luminescent centers and emit light through the radiative recombination of injected electrons and holes in the luminescent layer. Photoluminescent diodes use CDs as phosphors and emit different colors upon excitation with an external light source. The key technology for light-emitting devices is the preparation of high-quality carbon dot materials and optimization of the corresponding device structures.
-
Photo-induced LEDs are light-emitting devices that use ultraviolet or blue light chips as substrates and emit specific colors. Covering the substrate with a fluorescent film or powder that can absorb short wavelengths is mainly achieved by absorbing short-wavelength energy from the outside and converting it into long wavelengths to excite the CDs for luminescence. Using the PL properties of CDs, they can be used as active emitting layers in combination with ultraviolet or blue chip light pumping to prepare photoluminescent LEDs. Yang et al.80 used silver nitrate as a nitrogen source to enhance the blue-light emission of sodium-doped CDs through solid-state reactions. Research has shown that, compared with undoped CDs, the PL intensity of sodium–nitrogen co-doped CDs is increased by 5 times, with PL QYs reaching up to 24%. The improvement in the PL QYs is related to the elimination of non-radiative composite defects on the surface of the Na-CDs. A strong cold white LED with a maximum brightness of 4520 cdm−2 was successfully prepared by mixing yellow phosphor as a color conversion material with blue-emitting Na-CDs and placing it on a commercial UV LED chip with 365-nm emission. The color temperature was 5322 K, and the CIE coordinates were (0.33, 0.24). Dong et al.81 prepared copper-ion-doped CDs (Cu-CDs) by a one-step hydrothermal method. The band structures and luminescent centers of the Cu-doped CDs changed, resulting in Cu-CDs with blue, yellow, and red luminescent properties. At an excitation wavelength of 560 nm, the absolute QY of the Cu-CDs was 30%. Under 365-nm ultraviolet excitation, the multicolor Cu-CDs formed pure white luminescence at an appropriate synthesis ratio and successfully prepared a white light-emitting device (WLED) with CIE color coordinates of (0.337,0.337) and a color rendering index value of 89. Gong et al.49 synthesized red CDs (M-CDs) using manganese acetate as the doping agent. M-CDs and agar hydrogels were combined to form a composite material, which was coated on a 365-nm LED chip. High-performance monochromatic LED devices with CIE coordinates of (0.4884, 0.4237), a color temperature of 2635 K, and a color rendering index of 83.2 were prepared. At present, according to the preparation methods of CDs functional thin films and their roles in devices, they can be applied to LEDs in three ways: first, CDs can be doped with the main material as the luminescent layer; second, CDs can be used as the luminescent layer alone; and third, CDs can be used as the interface transport layer.
This study shows that the PL intensity of carbon dots is closely related to the temperature. Liu and team82 synthesized nitrogen-doped carbon dots and found that with increasing temperature, non-radiating channels were activated owing to thermal effects. Thermal activation of non-radiative channels at high temperatures intensifies the non-radiative recombination of electrons, weakens the hole strength, reduces the radiative recombination process, and thus reducing the PL strength.
-
Unlike photo-induced LEDs, electro-induced LEDs comprise a sandwich structure. Under the driving force of an external voltage, carrier radiative recombination achieves electroluminescence. Huang et al.83 reported the synthesis of high-concentration (9.8%) N-CDs using malic acid and o-phenylenediamine via solvothermal nitrogen synthesis (Fig. 5a). In Fig. 5b, the nitrogen doping in the carbon core structure reduces its bandgap width, and the electron donor groups of the nitrogen atoms greatly increase the π electron cloud density in CDs, thereby achieving efficient exciton radiative transitions, which is conducive to efficient electroluminescence. Therefore, the obtained N-CDs had a deep-blue emission at 415 nm, 60% high PL QY, and 1.74% EQE (Fig. 5f). The maximum brightness of the prepared CLED device was 1155.0 cdm−2, and the CIE (0.16, 0.08) is close to the standard color of HDTV Rec. BT.709 (0.15, 0.06), achieving deep-blue electroluminescence with high color purity. Wang et al.84 prepared CDs co-doped with oxygen and nitrogen using a hydrothermal method with an absolute PL QY of 88.9%. These CDs were doped into polyvinylcarbazole (PVK) and used as luminescent layers to prepare 2.114%-EQE pure blue CD-LEDs. The CIE coordinates (0.14, 0.10) were very close to the standard pure blue CIE coordinates (0.14, 0.08) specified by NTSC 1953, with a maximum brightness of 648 cdm−2.
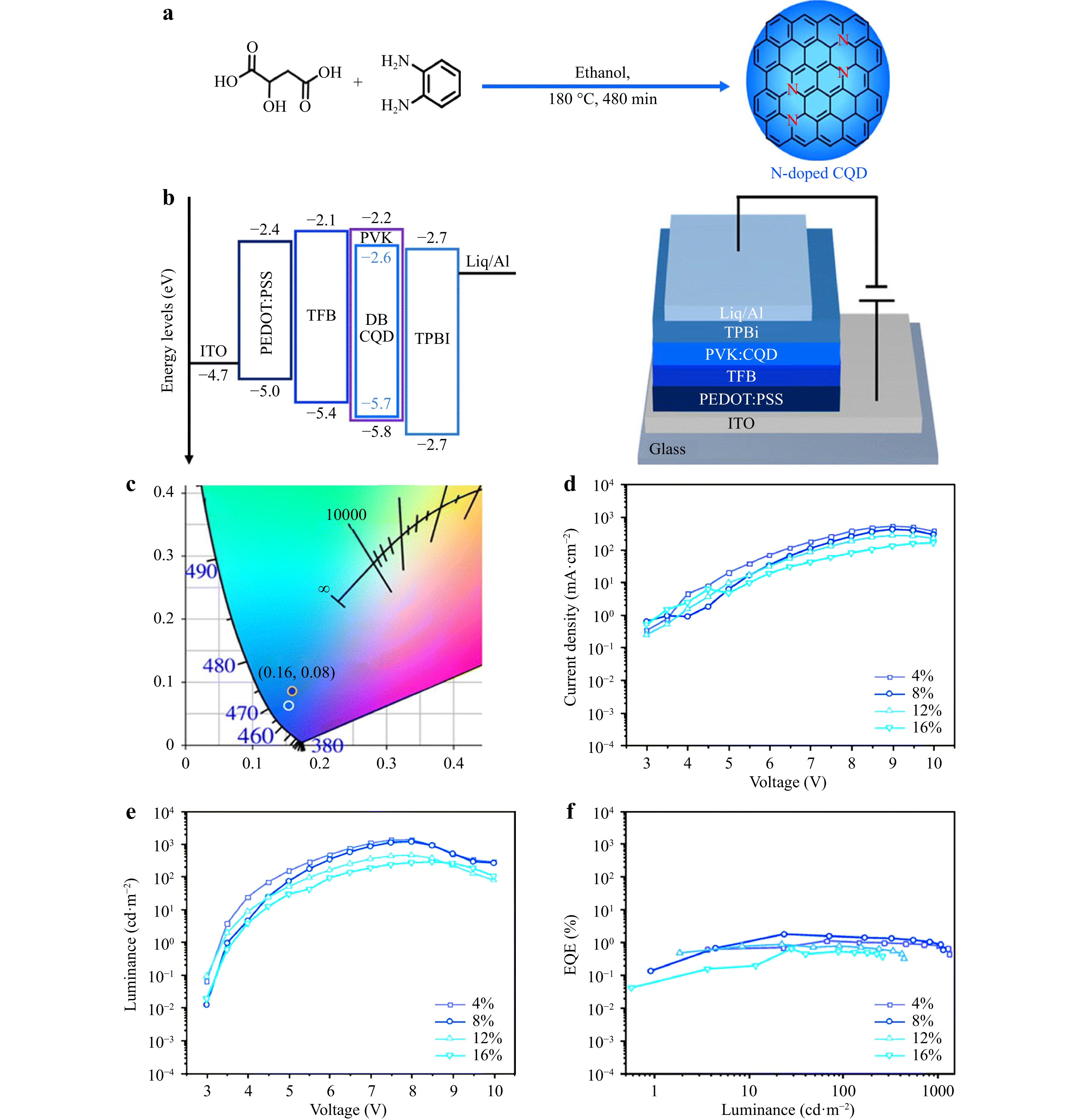
Fig. 5 a Synthesis route for N-CDs. b Energy diagram and structure of CLED. c CIE coordinates of 8%-CLED. d Current density and e luminance of LEDs as a function of applied voltage. f External quantum efficiencies of LEDs as a function of luminance. Reproduced with permission83 (Royal Society of Chemistry 2023, Chemical Communications).
-
Owing to their excellent electrical and optical properties, chemical resistivity, and low toxicity, CDs are considered to be efficient light-absorbing materials in the field of photovoltaics85. The energy difference between the LUMO and HOMO is called the HOMO–LUMO bandgap, which is the threshold for photon absorption and can affect the efficiency of optoelectronic device conversion. The bandgap can be altered in various ways, such as by applying an electric field or stress, changing the crystal and electronic structure of materials, and doping. Among these methods, doping is the most widely used. Kurukavak et al.86 synthesized B-CDs using boric acid as a boron source (Fig. 6a–c). Different concentrations of B-CDs were added to perovskite (MAPbI) solutions, which were made into thin films and used as active layers for solar cells. The addition of 5% vol. B-CDs can improve the crystallinity of the perovskite crystals, passivate the grain boundaries, and promote carrier migration. The photoconversion efficiency (PCE) increased from the initial 10.4% to 12.7%.
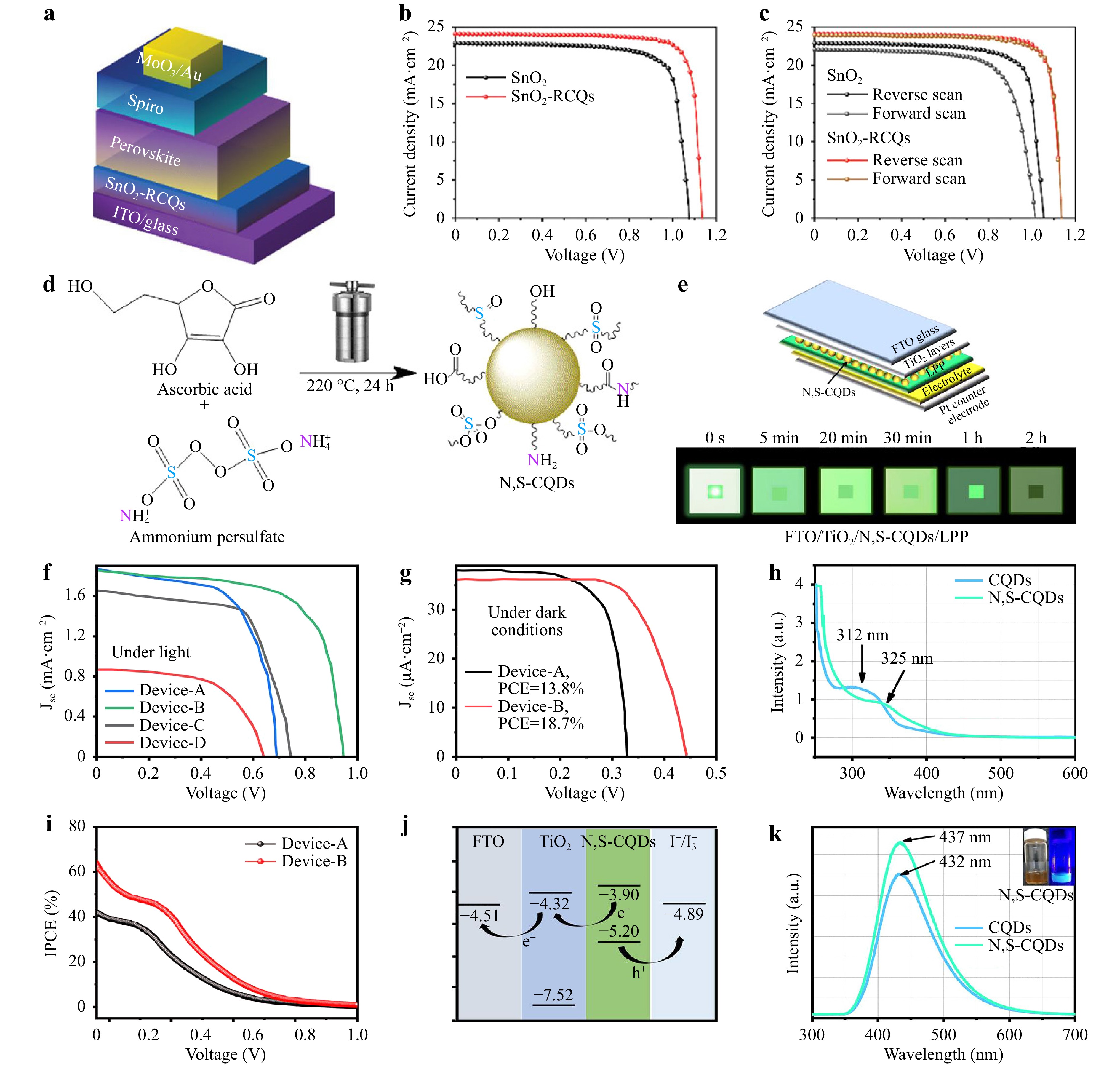
Fig. 6 a Schematic of the inverted planar heterojunction structure used for the PSCs. b, c Current density versus voltage (J–V) curves for champion PSCs. Reproduced with permission85 (Wiley 2020, Advanced Materials).d Synthesis of N, S-CDs. e Assembly of all-weather solar cell; J–V curves for All-weather solar cells. f Under simulated light irradiation; g Under dark conditions; h UV–Vis spectra of CDs and N, S-CDs; i IPCE spectra of representative devices. j Energy level distribution and charge transfer within a photoanode. k PL of CDs and N, S-CDs. Reproduced with permission87 (Elsevier 2023, Materials Today Energy).
Esakkimuthu et al.25 synthesized heteroatom-doped N-CDs, B-CDs, and F-CDs. Compared to other materials, N-CDs have a lower resistance, smaller bandgap, higher current density, and higher absorption owing to the binding of electron-rich CDs with polyaniline chains. Riaz et al.87 synthesized nitrogen–sulfur co-doped CDs using ascorbic acid and ammonium persulfate as the carbon, nitrogen, and sulfur sources, respectively. They also prepared all-weather solar cells (AW-SCs) composed of NS-CDs and green light storage materials (LPPs) combined with a dielectric layer of TiO2 (Fig. 6e), enhancing the optoelectronic performance of the devices under dark light conditions. The PCE of the AW-SC devices with the m-TiO2/N and S-CD/LLP photoanode structures increased to 18.7% in a completely dark environment (Fig. 6g). This is because doping nitrogen and sulfur into CDs reduces their bandgap and enhances charge injection into the TiO2 layer, thereby improving the photovoltaic performance of AW-SC devices. Ghadari et al.88 prepared nitrogen–chloride–co-doped CDs using choline chloride urea deep eutectic solvents. The NCl-CDs were used as cosensitizers for two phthalocyanines (MPc and MTCPc). Compared to pure MPc and MTCPc, the addition of NCl-CDs reduced the energy gap by approximately 2 eV. Compared to pure ZnTCPc, the addition of NCl-CDs increased Jsc from 1.99 to 9.87 mAcm−2. A smaller bandgap is beneficial for electrons to transition from the valence band to the conduction band, thereby improving the performance of the solar cells. In addition, Hezarkhani et al.89 applied N-CDs and metal–nitrogen co-doped CDs (MN-CDs; M: Mn, Fe, Co, Ni, Cu, and Zn) as co-sensitizers in the PSSC structure of sensitized solar cells. They found that ZnN-CDs improved the performance of the PSSC most significantly, with the PCE increasing from 1.84% to 3.09%. Zn2+ directly affects the HOMO and LUMO of the Zn-NCDs, reducing their bandgap. This work implied that CDs doped with heteroatoms can be used as additives, sensitizers, or active-layer materials to improve the photovoltaic performance of solar cells90.
In a study of MAPbI3 thin films, the charge traps formed by uncoordinated lead atoms on the surface of the thin films significantly affected their photoelectric characteristics. The carboxyl, hydroxyl, amino, and other functional groups can effectively interact with the lead atoms, thus significantly reducing the defect density of the perovskite films. In addition, CDs, as effective nucleation centers, not only promoted the crystallization of MAPbI3, but also significantly increased the grain size. These combined effects had a positive effect on the photoelectric properties of the perovskite films and improved their photoelectric conversion efficiency and stability91. Kurukavak et al.92 successfully synthesized P-CDs, which were introduced into perovskite precursor solutions with different volume ratios (1%, 3%, and 5%). After a systematic study, they found that compared to the control group without P-CDs, when the amount of P-CDs added was 1 and 3 vol%, respectively. The crystal sizes of perovskite films increased significantly to 82.89 and 71.55 nm, respectively (69.17 nm in the control group). The results show that the introduction of P-CDs effectively promotes the crystallization process of perovskite, increasing the grain size and significantly reducing the grain boundary density and carrier recombination in the perovskite films. Therefore, the photoelectric properties of the perovskite films improved.
-
Medical imaging is one of the most effective methods for disease diagnosis and monitoring. Unfortunately, each imaging technique has different disadvantages in terms of sensitivity, resolution, and pathological change screening; therefore, multiple detection methods are sometimes used to assist in diagnosis. Therefore, many studies have focused on improving the performance of detection methods to meet the needs of medical diagnoses as much as possible. Various cell types possess distinct structures, morphologies, and biomarkers within their membranes or cytoplasm, resulting in unique reactions with foreign carbon nanoparticles. CDs possess many outstanding characteristics, including excellent biocompatibility, photoluminescence, low toxicity, affordability, ease of acquisition, small volume, and high QY. The foremost advantage of carbon nanoparticles lies in their ability to enhance imaging capabilities, causing them to be used extensively in the field of imaging and hold immense potential in diagnostics. Based on this, CDs with specialized functions were synthesized, providing opportunities for the utilization of CDs for in vivo biological imaging. Zhang et al.93 synthesized N-CDs using citric acid as a raw material and propylene diamine as a passivation agent. The surfaces of the N-CDs are composed of carboxyl and amino groups, which facilitate their entry into cells. Thus, N-CDs were used to stain HeLa cells, and fluorescence microscopy showed that the N-CDs were located in the nucleus and exhibited a multi-colored luminescence effect, whereas there was almost no blue-green-red fluorescence intensity in the cytoplasm. The N-CDs automatically accumulate in the nucleus, resulting in very low concentrations in the cytoplasm. N-CDs accumulate in the nucleus and bind to chromatin, with their light intensity varying according to changes in chromatin concentration. This emerging biological imaging tool is promising for exploring nuclear alterations throughout the cell cycle. Wang et al.94 synthesized N-CDs using citric acid monohydrate and glutathione as raw materials. Owing to the low cytotoxicity and good biocompatibility of the synthesized CDs, the N-CDs can be successfully used as fluorescent probes for the imaging of HepG2 cells. Wang et al.95 prepared Cu-CDs with a fluorescence QY of up to 24.4% by coordinating copper ions with carboxyl groups using polyacrylic acid copper complexes as raw materials (Fig. 7a). Because of their excellent solubility, high fluorescence intensity, and low cytotoxicity, Cu-CDs have been successfully used for the fluorescence imaging of HeLa (human cervical cancer) cell lines and SH-SY5Y (human neuroblastoma cells) multicellular spheres (Fig. 7c).
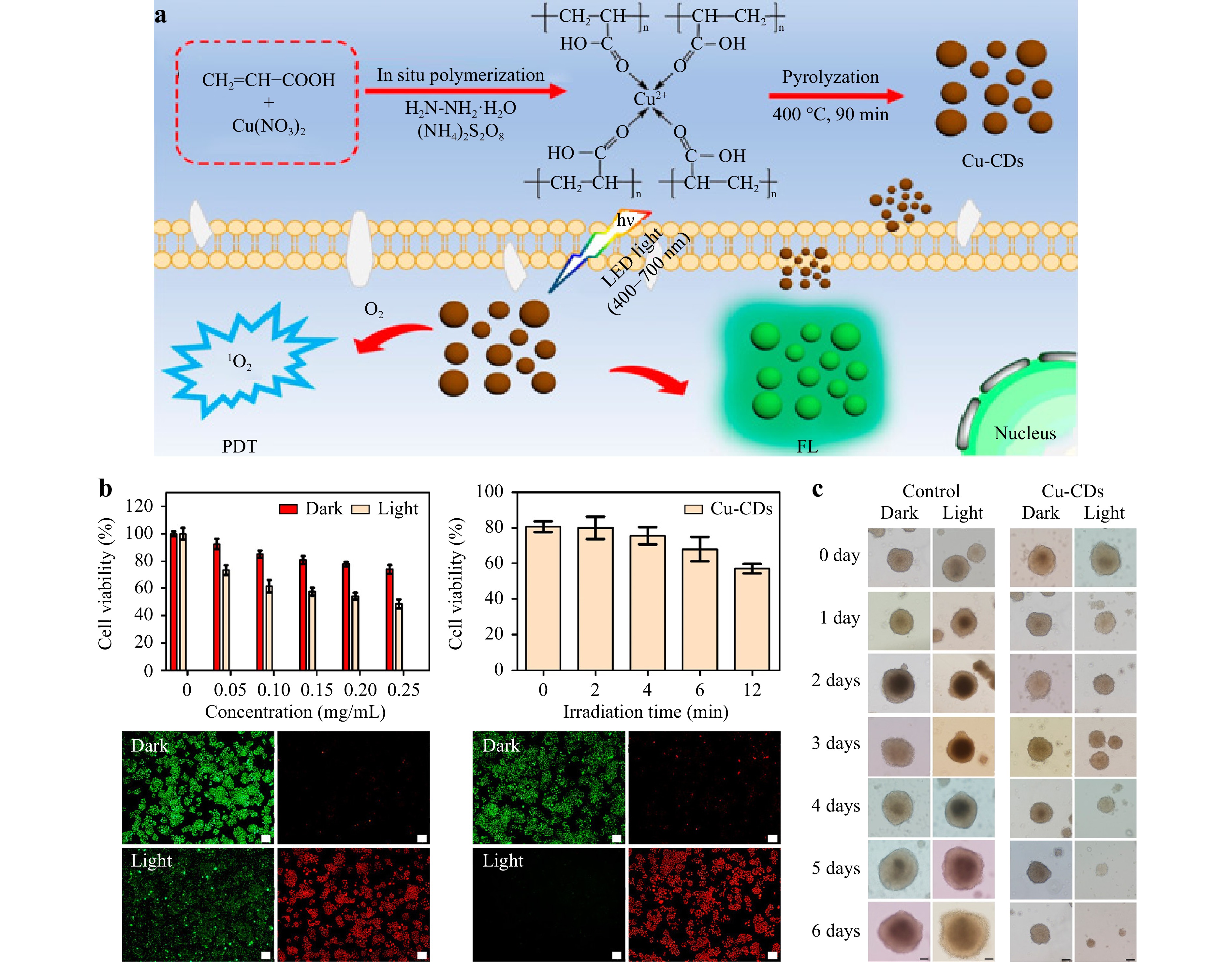
Fig. 7 a Schematic of the synthesis of the Cu-CDs. b Cu-CD performance and imaging. c Images of SH-SY5Y 3D MCs co-incubated with DMEM and Cu-CDs captured by optical microscopy over six days. Reproduced with permission95 (American Chemical Society 2019, Inorganic Chemistry).
-
Bioimaging technology is widely used in clinical research and practice to observe and study biological reactions in cells, subcellular organisms, and small animals89. Doping CDs can improve their imaging ability and enable exploration of their application prospects in the field of biological imaging. Zheng et al.96 synthesized Pn-CDs and highly fluorescent NPn-CDs using a hydrothermal method. Compared to pure Pn-CDs, NPn-CDs exhibited better stability and superior optical properties owing to the incorporation of nitrogen; thus, they can be applied as fluorescent dyes for microbial imaging. Research has found that prepared NPn-CDs can be effectively applied in the in vivo imaging of Escherichia coli, Bacillus subtilis, brewing yeast, onion inner epidermal cells, paramecium, and animals. This indicates that NPn-CDs are biocompatible and nontoxic nanofluorescent materials, making them ideal contrast agents for biomedical applications. Zhu et al.97 synthesized several multi-element doped CDs using o-phenylenediamine, sodium phosphotungstate octahydrate, and 0.6/0.8 g hafnium chloride, including phosphorus–tungsten co-doped CDs, phosphorus–tungsten–hafnium co-doped CDs (0.6 g), and phosphorus–tungsten–hafnium co-doped CDs (0.8 g). Comparing these three co-doped CDs, it was demonstrated that PWHf-CDs can ensure aggregation and effective retention at the tumor site and have significant advantages in tumor imaging, tracking, and biosafety.
-
Magnetic resonance imaging (MRI) can be used to detect and diagnose diseases of the heart, blood vessels, and thoracic and abdominal organs and is widely used to track the condition and function of tumors. CDs have excellent physical and chemical properties, such as multicolor luminescence, low cytotoxicity, and excellent biocompatibility; therefore, X-CDs can be used as contrast agents for MRI, enhancing imaging effects and facilitating medical diagnosis. Ramos et al.76 synthesized CDs co-doped with nitrogen and lanthanide elements (Gd and Yb) using ethylenediamine, citric acid, and two lanthanide salts as precursors. The results of in vitro and in vivo studies showed that NGdYb-CDs can serve as contrast agents in MRI techniques and provide higher image contrast. Jiang et al.98 reported the preparation of novel Gd-CDs using gadolinium chloride as a dopant. Their results indicate that Gd-CDs can serve as T1 contrast agents and improve MRI performance by increasing the longitudinal relaxation rate of the surrounding water molecules. Compared with the most used paramagnetic contrast agents, Gd-CDs display brighter and clearer fluorescence and better effects in clinical practice. Therefore, X-CDs are valuable for MRI, and many studies have been dedicated to improving the performance of contrast agents.
-
CDs are fluorescent nanomaterials that exhibit strong fluorescence emission under single-photon and multiphoton excitation and have low toxicity. Therefore, their fluorescence characteristics can be used to detect metal ions, pollutants, and other environmental substances. Li et al.99 synthesized two fluorescent CDs and mixed them in equal mass ratios. This study demonstrated that mixed CDs can be used for the detection of Hg+. Through interaction with oxygen-containing groups, mercury ions promote the aggregation of CDs, thus triggering the non-radiative recombination of excitons, which ultimately leads to weakening of the fluorescence intensity. Wang et al.100 summarized the applications of CDs in cancer detection. Many studies have reported that CDs can be used as fluorescent probes or sensors for the detection of targeted substances. Their detection selectivity and sensitivity have attracted widespread attention and they have significant application prospects in the field of detection. The detection principle involves fluorescence quenching or enhancement. Specific metal ions and pollutants can cause a sudden decrease or even disappearance of the CD fluorescence intensity, thereby facilitating detection. Raju et al.19 prepared P-CDs with a fluorescence QY of 16.1% via a hydrothermal method using trisodium citrate and phosphoric acid and found that Cu2+ produces the more OH free radicals through reaction with H2O2 than the other metal ions that were examined, and enhances luminescence intensity. Therefore, P-CDs are suitable for the selective detection of Cu2+.
-
The working processes in industries such as mechanical manufacturing, chemical engineering, electronics, mining, and electroplating generate many heavy-metal ions. Heavy-metal ions have high toxicity, accumulation, and non-degradability, which not only pollute the environment but also endanger human health. Therefore, green and effective methods for the detection of heavy metals must be developed. Fluorescence detection technology has the advantages of strong selectivity, fast reaction, and high sensitivity, and can detect heavy metals.
Zhang et al.101 used a green raw material from Chinese medicine residue as a carbon source to synthesize B-CDs using the solvothermal method. The addition of 12 metal ions to a solution containing B-CDs and comparison of their fluorescence intensities showed a sharp decrease in fluorescence intensity after adding Fe3+, whereas the influence of the other metal ions was weak. This indicates that the B-CDs exhibited higher selectivity towards Fe3+. Song et al.102 prepared M-CDs using glutathione, diethylenetriamine, and ammonium ferric citrate as sulfur, nitrogen, and iron sources. To evaluate the specificity, 14 ions were exposed to probe solutions containing M-CDs. The results showed that only the addition of Hg2+ caused a sudden decrease in the fluorescence intensity, whereas the addition of other ions had little effect on the fluorescence intensity, as shown in Fig. 8a. Molkenova and Atabaev103 prepared P-CDs with glucose and disodium hydrogen phosphate and used them for ion detection. Compared with other metal ions, Fe3+ ions caused significant fluorescence quenching (approximately 87%) of P-CDs owing to the non-radiative recombination of excitation electrons with Fe3+ ions. The strong fluorescence change indicated that the P-CDs had a high degree of selectivity for Fe3+ ions. Preethi et al.104 used raw rice and phosphoric acid as the carbon and phosphorus sources, respectively, to synthesize efficient, nontoxic, and environmentally friendly P-CDs (Fig. 8b). When Mn3+, Fe2+, Al3+, Pb2+, Ca2+, Fe3+, Cu2+, Hg2+, Mg2+, Co2+, and Zn2+ were added to the P-CD solution, Cu2+ ions exhibited the lowest quenching rate, indicating that P-CDs could be used to detect Cu2+. In addition to detecting Fe3+, Hg+, and Cu2+, P-CDs can also be used as fluorescent sensors for detecting metal ions such as Al3+105, Cr2O72−106, Pb2+107, Cd2+108, and Au2+109. Currently, most CD materials exhibit the detection of a single ion, and little research exists on multifunctional detection and fluorescent materials that can simultaneously detect and remove ions.
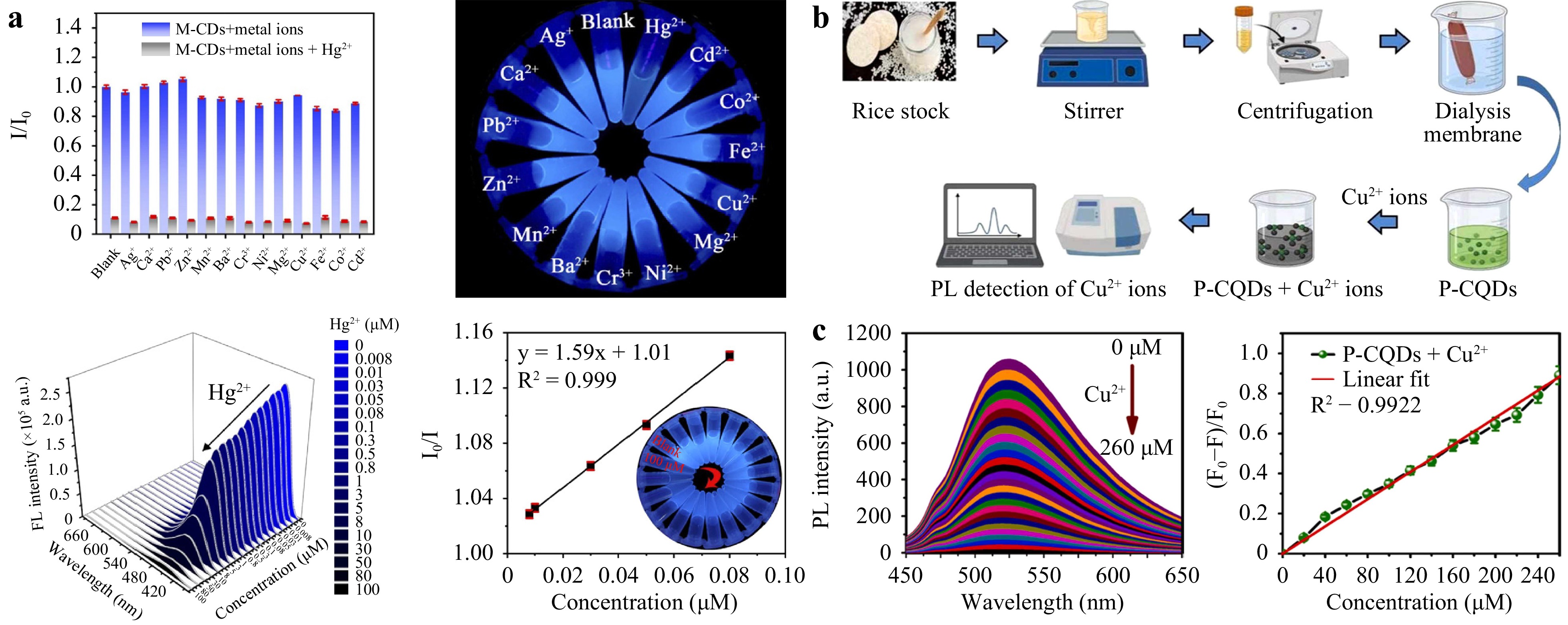
Fig. 8 a M-CD performance. Reproduced with permission102 (Elsevier 2023, Journal of Hazardous Materials). b Schematic of the synthesis and detection of Cu2+ ions in P-CDs. c P-CD detection of metal ions. Reproduced with permission104 (Elsevier 2022, Colloids and Surfaces A: Physicochemical and Engineering Aspects).
-
In addition to heavy-metal ions, CDs can also be applied in other detection fields, including, but not limited to, the detection of pollutants such as furazolidone110, specific nitrofuran antibiotics111, aflatoxins112, and pesticides. Ma et al.113 combined Fe-CDs with metal–organic frameworks (MOF-808) to prepare a multifunctional nanoreactor, successfully achieving sensitive detection and selective degradation of oxygen, phosphorus, and parathion in pesticides. Li et al.22 successfully prepared N-CDs with specific properties and added them to a conventional colorimetric ELISA system to establish a label-free fluorescence assay for detecting trace amounts of cefuroxime residues in milk. The sensitivity of the determination can be directly increased by a factor of approximately 4–10 times. Babar and Garje114 reported the preparation of NP-CDs using glucose as the carbon source, liquid NH3 as the nitrogen source, and P2O5 as the phosphorus source. The synthesized NP-CDs were insoluble in water, but after treatment with concentrated nitric acid, they were functionalized and became water-soluble NP-CDs (wsNP-CDs). The addition of nitroexplosive TNP to the wsNP-CD solution significantly reduced fluorescence intensity. During excitation, the wsNP-CDs emitted fluorescence. When TNP was present in the solution, the wsNP-CDs transferred the fluorescence resonance energy to TNP, which absorbed the energy and underwent quenching. The wsNP-CDs exhibited excellent fluorescence and were successfully used to detect nitro groups. Yin et al.115 used uric acid and diethylenetriamine as carbon and nitrogen sources, respectively, to synthesize N-CDs using a one-step method. Their study found that the fluorescence of N-CDs can be quenched by mercury ions (Hg2+). Because of the strong coordination between the carbon–nitrogen heterocycle of melamine and Hg2+, when melamine is mixed with Hg2+, some Hg2+ ions coordinate with melamine. Subsequently, the fluorescence of the N-CDs was quenched using the remaining Hg2+ to determine the concentration of melamine. This shows that N-CDs can be used as fluorescent probes to detect melamine.
-
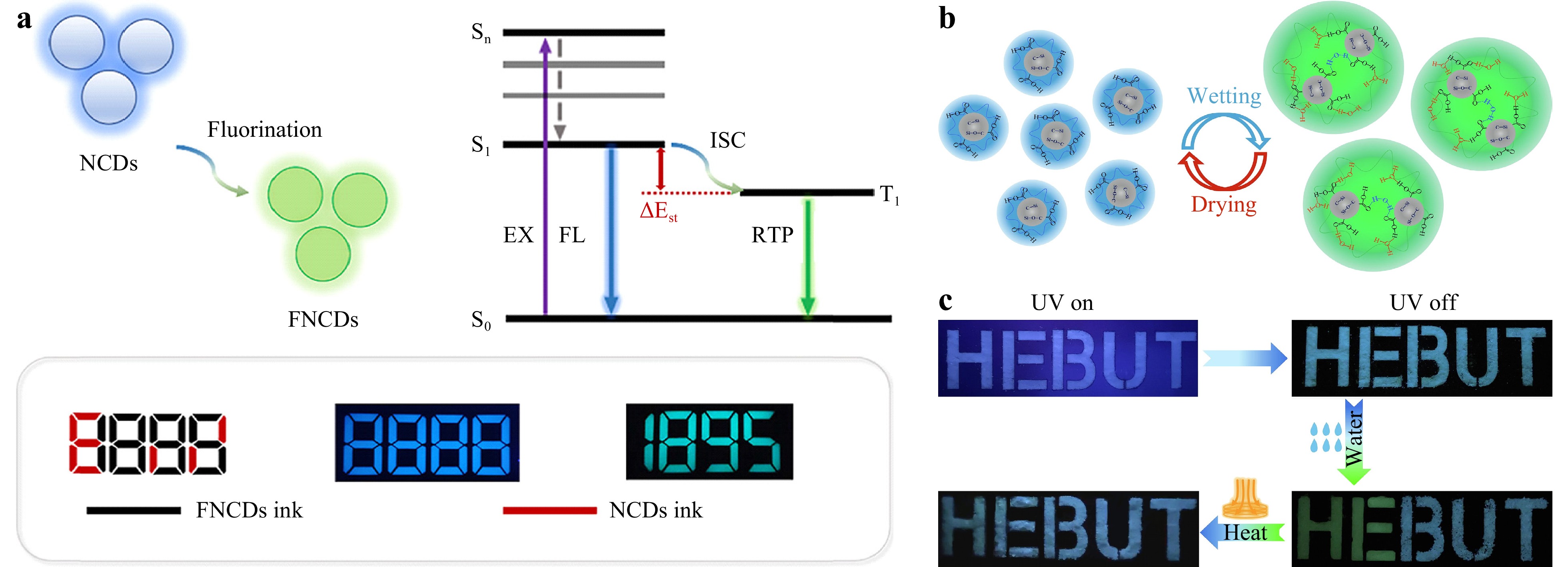
In the information age, counterfeit goods and false information are constantly emerging, and pose potential threats to social stability and security. To address this issue, it is necessary to develop safer, more stable, and more precise anti-counterfeiting technology116. Fluorescent anti-counterfeiting offers strong concealment and is often considered an ideal anti-counterfeiting technology. Many studies have focused on the development of easily recognizable but difficult-to-forge encrypted anti-counterfeiting fluorescent materials. As we have established, CDs are nanomaterials with fluorescent properties and are inexpensive and easily obtainable, with enormous optical anti-counterfeiting and encryption potential. Hang et al.117 used silicon as a doping element to form Si-C covalent bonds in CDs. Water was used to regulate the dynamic network of Si-C and hydrogen bonds, resulting in the transition from a blue to green afterglow (Fig. 9b). In the presence and absence of water, a hydrogen bond network between CDs and Si forms and breaks, allowing for reversible multilevel information encryption applications through alternating water and heating. Liu et al.69 prepared NF-CDs through gas-phase fluorination and printed them as an anti-counterfeiting ink on filter paper using a commercial inkjet printer. Under 365-nm ultraviolet light, bright letters can be seen (Fig. 9a). After the UV lamp was switched off, the light signal was visible to the naked eye for more than 5 s. This confirms that the NF-CD ink can be used as an advanced anti-counterfeiting ink with a high resolution.
-
This article summarized and introduced the research and applications of metal and nonmetal element-doped CDs in recent years, with a focus on analyzing the mechanisms of heteroatom doping affecting the photophysical properties. Owing to the overlap of atomic orbitals between heteroatoms and carbon atoms, as well as the electronic effects of heteroatoms, introducing heteroatoms into the structure of CDs may effectively alter their electronic structure, nanostructure, and chemical composition. The sizes of nonmetal atoms are similar to that of carbon atoms, making it easy to realize effective and uniform doping. Compared to non-metal heteroatoms, metal heteroatoms have more unoccupied outer orbitals and more opportunities to lose electrons, resulting in more significant changes in the physical and chemical properties of doped CDs. Doping CDs with metal ions can effectively alter the charge density and charge transformation form between the metal ions and CDs by combining them with the excellent electron mobility of CDs, thereby significantly regulating their physical and chemical properties. Metal ions optimize the optical properties by modulating band structures and introducing new functional groups, which may induce CDs to exhibit new physical and chemical properties, such as catalytic properties. The improved properties of X-CDs make them applicable to optoelectronic devices, information encryption, anti-counterfeiting, imaging, detection, and other fields.
Although metal and non-metal doping can effectively regulate the properties of CDs, there are still some challenges in the study of X-CDs. First, it is difficult to achieve precise doping, such as controlling the doping at the edges or centers of CDs. Second, the quantitative control of the doped atoms in CDs is difficult. Third, the size of the doped atoms is difficult to control. The development of standardized methodologies for the synthesis, purification, and precise structural characterization of CDs is crucial. Further advancements are critical for enhancing the accuracy of the heteroatom doping ratios. Elucidating the synthesis pathway between the precursor units may pave the way for precise doping and serve as a valuable guide. Although current research has proposed some possible fluorescence mechanisms, these mechanisms are not yet unified, including the effect of doping on PL, which has not been fully resolved. Therefore, there is an urgent need to combine theoretical and experimental studies and adopt corresponding characterization methods to accurately describe the mechanism of action of PL and the effect of doping on it. In addition, the development of metal-doped CDs lags behind that of non-metal-doped CDs, especially for the application of CDs in luminescent devices. Further research on their mechanisms and material designs is of great importance to improving their performance and expanding their application potential.
-
This work was financially supported by the National Key Research and Development Program of China (2019YFE0112200) and the Science and Technology Development Fund of Macau SAR (0128/2020/A3).
Metal and non-metal doped carbon dots: properties and applications
- Light: Advanced Manufacturing 5, Article number: (2024)
- Received: 04 January 2024
- Revised: 25 July 2024
- Accepted: 26 July 2024 Published online: 22 October 2024
doi: https://doi.org/10.37188/lam.2024.041
Abstract: Carbon dots (CDs) have shown great potential for application in optoelectronics, owing to their merits of tunable fluorescence, biocompatibility, low toxicity, and solution processability. However, the intrinsic nature of CDs makes them prone to fluorescence quenching in the aggregated state. In addition, the emission peak width at half maximum of a single CD is usually greater than 60 nm, and the emission spectra may exhibit a multi-peak superposition state, resulting in poor monochromaticity. Further, the unsatisfactory quantum yield of CDs restricts their further application. Considering this, doping strategies have successfully improved the electrical, optical, and chemical properties of CDs. The intrinsic structure and electron distribution of CDs can be effectively adjusted by metal or nonmetal doping. Doping atoms generate n- or p-type charge carriers, changing the bandgap energy, and thereby improving the photophysical properties of the CDs. In this comprehensive review, we explore the intricate effects of various doping strategies on CDs and systematically categorize them. Notably, we elaborate on the diverse types of doped CDs and emphasize their photophysical properties, aiming to elucidate the fundamental mechanisms underlying the influence of doping on CD performance. Specifically, this review describes the extensive applications of doped carbon dots (X-CDs) in optoelectronic devices, information encryption, anti-counterfeiting measures, imaging techniques, and detection fields, to spur further X-CD exploration and application.
Research Summary
Metal and non-metal doped carbon dots: properties and applications
Carbon dots (CDs) exhibit great potential in optoelectronics due to their unique properties. However, they face challenges such as fluorescence quenching, poor monochromaticity and low quantum yield. To overcome these limitations, doping strategies have been successfully applied to improve the optical properties of CDs. Through metal or non-metal doping, the intrinsic structure and electron distribution of CDs can be effectively regulated, thereby altering their bandgap energy and optimizing their photophysical properties. This review explores the intricate effects of various doping strategies on CDs, categorizes them systematically, and elaborates on diverse doped CD types, emphasizing their photophysical properties to elucidate the fundamental mechanisms of doping influence. Specifically, it describes the extensive applications of doped carbon dots (X-CDs) to spur further exploration and application.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article′s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article′s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.






 DownLoad:
DownLoad: