-
Attaining sub-diffraction-limit resolution in thick, scattered biological tissues remains a pivotal challenge in optical microscopy. In a recent publication in Light: Science & Applications, Jo et al. presented a notable advancement: a hybrid-multiplexed multifocal metalens engineered for image scanning microscopy (ISM) that achieved high-resolution, deep-tissue imaging of brain organoids within a compact optical system (Fig. 1)1.
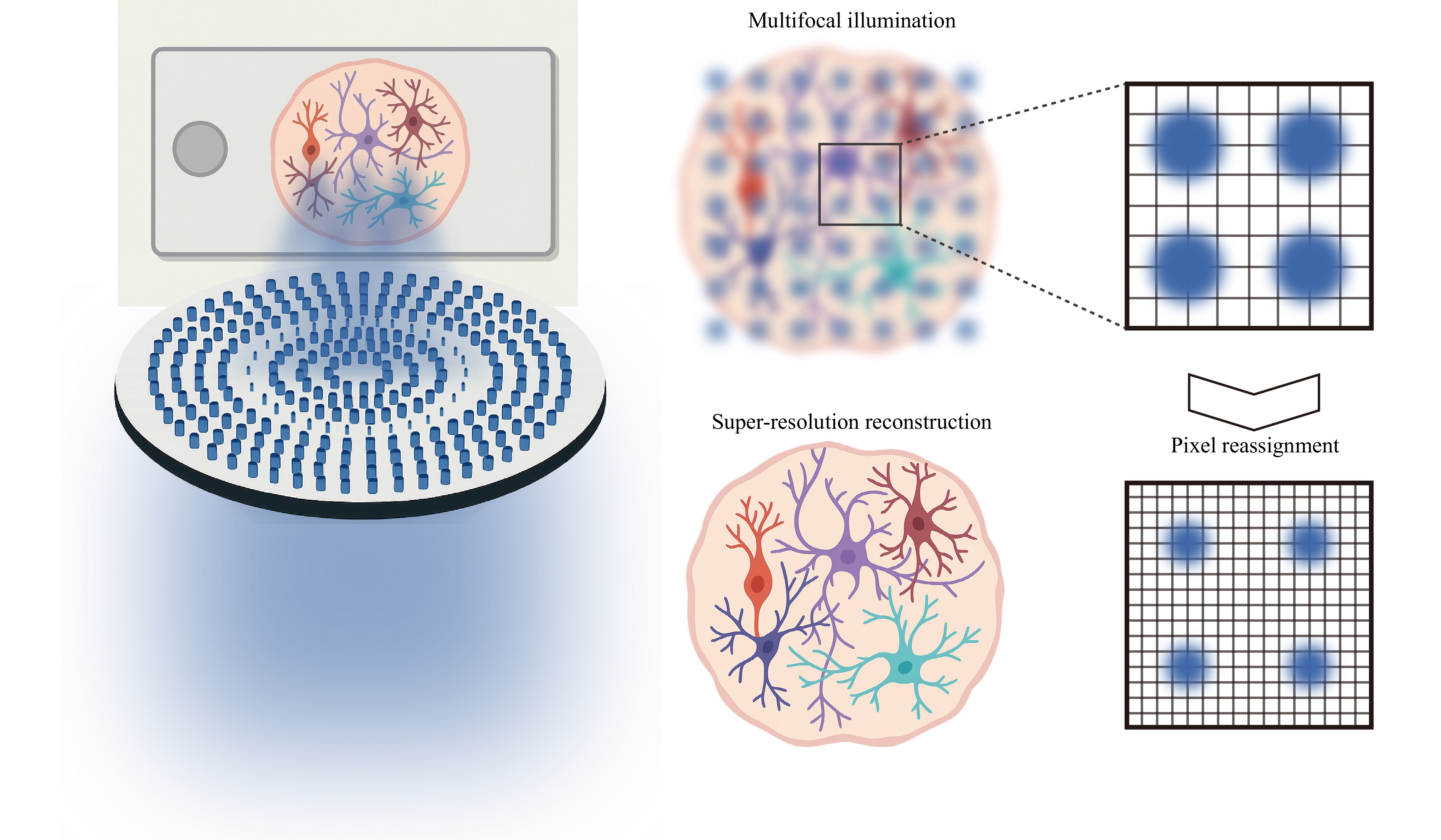
Fig. 1 Illustration of the multifocal metalens (left) and pixel reassignment reconstruction (right).
ISM improves spatial resolution by replacing the single-point detector of traditional confocal systems with an array detector, allowing fine sampling of individual excitation spots. Through pixel reassignment and subsequent deconvolution, ISM can realise up to a two-fold enhancement in resolution beyond the diffraction limit2,3. To boost temporal resolution, multifocal illumination schemes are utilised to parallelise the scanning process4,5, making ISM ideal for high-speed and volumetric imaging in biological applications. Numerous strategies have been developed to generate multifocal excitation, including digital micromirror devices (DMDs) and microlens arrays (MLAs). However, these approaches face limitations due to trade-offs between cost, numerical aperture (NA), pitch, and multifocal uniformity. Metalenses—ultrathin nanostructured surfaces capable of precise phase modulation—present a promising alternative. Their design flexibility and compact form factor complement traditional methods such as MLAs, pinhole arrays, beam-splitting prisms, spatial light modulators (SLMs), DMDs, and diffractive optical elements (DOEs). Table 1 offers a comparative summary of various multifocal generation techniques.
Category Method Advantage Limitation References Traditional optics Microlens array Simple structure; efficient multifocal excitation. Low flexibility; non-uniform illumination. 6,7 Pinhole array Low-cost fabrication; robustness to optical aberrations. Low optical throughput. 5 Beam-splitting prism High power efficiency. Precise alignment required,
lack of tunability.8 Dynamic modulators Spatial light modulator Real-time programmability;
flexible focal control.Polarisation sensitivity; wavelength dependence; limited speed. 9,10 Digital Micromirror Device Rapid pattern switching;
dynamic control.Limited phase modulation;
low optical efficiency.4 Diffractive/Nanostructured Diffractive optical element Customisable layouts design. Static configuration; fabrication sensitivity. 11 Metalens/Metasurface Compact form factor; design versatility. Narrow spectral bandwidth; complex nanofabrication. 1 Table 1. Comparative summary of different multifocal generation strategies.
To overcome the drawbacks of conventional methods, Jo et al. developed a hybrid multiplexing technique that combines two existing approaches: phase addition and random multiplexing1. By splitting the desired focal pattern into interleaved even and odd subarrays, random multiplexing was independently applied using distinct random binary masks to reduce interference between neighbouring foci. The resulting phase profiles were then merged through simple addition, enabling the formation of a dense and well-isolated multifocal pattern. This method generates a 40 × 40 focal array with a 3-µm pitch and 0.7 NA, fabricated on a silicon nitride platform optimised for 488-nm excitation.
To demonstrate the practical application of their design, the authors integrated a metalens into a custom-built ISM platform termed multifocal metalens-based ISM (MMISM), which they used to image fluorescently labelled human forebrain organoids with up to 40 µm thickness. The system achieves neuronal feature resolution of approximately 330–370 nm—nearly twice that of widefield microscopy—while effectively suppressing out-of-focus background through the digital pinholing process.
Building on the principle that orthogonally polarised light beams do not interfere, Jo et al. extended their method to polarisation hybrid multiplexing, enabling the generation of denser and more uniform focal arrays1. This innovation not only increases focal density without inducing crosstalk but also reduces the number of required scanning frames, facilitating faster imaging. Additionally, the technique holds promise for future applications in chiral-selective imaging, where polarisation is crucial.
Combined with scalable fabrication methods like nanoimprint lithography and adaptability to multiphoton excitation for deeper imaging, the approach maps a clear path toward practical, high-throughput 3D imaging in organoid and tissue systems. Jo et al. emphasised that their hybrid multiplexing strategy extends beyond multifocal metalenses, offering a versatile framework for integrating arbitrary phase profiles in multifunctional metalens designs1. This flexibility allows multiple optical functions to be incorporated into a single compact device, simplifying system architecture. Recent advancements, such as the s2ISM introduced by Zunino et al.12, which computationally removes out-of-focus signals, and the C2SD-ISM developed by Liang et al.13, which physically eliminates the defocused background using a spinning disk, have maintained the super-resolution capabilities of ISM while significantly enhancing optical sectioning in thick or scattering samples. MMISM could significantly enhance its imaging performance by integrating these cutting-edge techniques.
Multifocal metalenses drive super-resolution imaging of brain organoids
- Light: Advanced Manufacturing , Article number: (2025)
- Received: 07 July 2025
- Revised: 11 August 2025
- Accepted: 20 August 2025 Published online: 24 October 2025
doi: https://doi.org/10.37188/lam.2025.071
Abstract: The generation of dense and uniform multifocal illumination patterns is essential for achieving rapid and artifact-free image scanning microscopy. Jo et al. introduced an innovative metalens design approach that facilitates the creation of low-pitch, uniform, and high-NA multifocal patterns, experimentally confirming its efficacy on biological samples.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article′s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article′s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.






 DownLoad:
DownLoad: