-
THz technologies have been vigorously explored during the past few decades1−14. Today, they offer a variety of applications in the non-destructive evaluations of composite and ceramic materials15,16, and semiconductor devices17−19, quality control in pharmaceutical20, and food21 industries, as well as public security tasks22−24. Among all THz applications, special attention is paid to the label-free medical diagnostics of malignant and benign neoplasms with different nosologies and localizations25−28, cerebral ischemia29, Alzheimer’s disease30,31, myelin deficit32, and traumatic injuries33,34 of the brain, thermal burns35, ophthalmological disorders36−39, caries and teeth demineralization40,41, diabetic foot syndrome42,43, monitoring of blood glucose level44,45, hydration46,47 and viability of tissues48, transdermal drug delivery49,50, as well as therapy of cancers and inflammatory diseases51,52. The majority of these applications involves measurements and analysis of the THz optical properties of an analyte.
Despite THz technologies becoming ubiquitous, their translation into all these practical fields is hampered by the lack of commercially-available THz endoscopes and related method to study hard-to-access objects25, 26. In contrast to the visible and infrared (IR) ranges with diverse fiber optics technologies and, thus, a variety of endoscopic systems53−56, in the THz range, a lack of such systems is evident, which is attributed to the nascent state of endoscopy hardware and methods for endoscopic data processing and analysis25, 57, 58.
In this review, we consider recent attempts to address the aforementioned problems of THz endoscopy, with an emphasis on THz applications in the medical diagnosis of neoplasms. For this, we overview important features of the THz-wave–tissue interactions, as well as the THz applications in medical diagnosis of benign and malignant neoplasms (Sec. 2), which helps us to better highlight the problems of THz endoscopy. Then, we discuss the two distinct approaches to mitigate them:
● The first uses a pair of fiber-coupled THz PCAs (or a single THz transceiver) to emit and detect THz waves in close proximity to a hard-to-access object (Sec. 3). In such a case, optical fibers are used to flexibly deliver the near-IR laser pump and probe beams to the THz emitter. For such systems, data acquisition and processing (including quantification of the THz optical properties of an analyte) are similar to those in THz pulsed spectroscopy and imaging.
● The second exploits flexible THz optical fibers (or, at least, hard THz waveguides) as a key element to deliver the THz waves from an emitter to an object and then to detect the reflected and back-propagated THz signal (Sec. 4). In such a case, the key technology relies on THz optical fibers and waveguides which are still rare, expensive, and inefficient. Despite the lack of commercially available elements of THz fiber optics and related methods to collect the THz data and retrieve the THz optical properties of an analyte, the recent developments pave the way for solving this problem.
We discuss several notable examples of THz endoscopic systems with their advantages and drawbacks. We point out prospects for further research and development in this demanding branch of THz technology, as well as a potential of THz endoscopy in medical applications (Sec. 5).
-
THz technology finds applications in different fields of modern science and technology, while its use in medical diagnosis and therapy is of particular interest59, 60. Among all medical applications, special attention is paid to the label-free diagnosis of malignant and benign neoplasms with different nosologies and localizations25−27, as well as the cancer therapy51, 52.
-
The considerable interest in the biomedical applications of THz waves is driven by the specific character of the THz-wave–tissue interactions, as compared to radiation from other bands of the electromagnetic spectrum59.
The THz waves are strongly absorbed by tissue water in free and bound states59, 61. On the one hand, this makes the THz waves sensitive to the content and state of tissues water, which is an important endogenous label of different pathologies, including malignant and benign neoplasms. On the other, this limits the depth of THz-wave penetration in tissues by ~ 10-100 μm depending on the frequency $ \nu $ and the tissue type. This allows probing only the superficial tissues by THz waves, which considerably reduces the useful range for THz biomedical applications.
Elements of biological tissues (i.e., cell organelles, separate cells and their agglomerates, microfibrils, and others) are commonly considered to be small at the scale posed by the THz wavelengths ($ \lambda \sim 100 $ μm–1 mm). This makes it possible to assume that tissue is optically homogeneous at the THz wavelength scale and, thus, leads to the ubiquitous use of the effective medium theory (EMT) to describe the THz-wave–tissue interactions25, 59, 62. Despite recent evidence of the THz-wave scattering in tissues, including the direct visualization of mesoscale ($ \sim \lambda $) THz-wave scatterers in tissues via the super-resolution THz microscopy63−67, theoretical and experimental studies of the parameters of such scatterers25, 68, observations and analysis of the polarization effects69−71 and THz birefringence of tissues72, EMT still dominates in THz biophotonics.
Within EMT, different models of complex dielectric permittivity are applied to describe the response of biological tissues and liquids in the THz range. Since liquid water is a dominant factor underlying the dielectric response of tissues, the THz response of tissues is described by relaxation-like models of complex dielectric permittivity59. Such models commonly comprise two or more Debye relaxators62, overdamped oscillators73, or other similar kernels, which describe broad absorption bands with no resonant spectral features. The parameters of such models for some healthy and pathological tissues are summarized in Refs. 25, 51.
-
For the differentiation between intact tissue and pathology using THz waves, one usually measures and compares the absolute values of their effective THz dielectric constants, that can be represented in the form of complex dielectric permittivity $ \tilde{\varepsilon} = \varepsilon' - i \varepsilon'' $, or complex refractive index
$$ \tilde{n} = n - i n'' = n - i \frac{ c_{\rm{0}} }{ 2 \pi \nu } \alpha = \sqrt{ \tilde{ \varepsilon } } $$ (1) where $ n $ is the real part refractive index, $ \alpha $ is the absorption coefficient (by field) in [cm−1] (notice, $ \alpha $ should be multiplied by a factor of 2 to obtain the power absorption coefficient–i.e., $ \alpha_{\rm{p}} = 2 \alpha $), and $ c_{\rm{0}} = 3 \times 10^{8} $ m/sec is the speed of light in free space. Here, $ n $ and $ \alpha $ are also referred to as the effective optical properties/constants. The quantified THz response of different tissues is statistically analyzed to uncover the distinction between them and to analyze the specificity, sensitivity, and efficiency of medical diagnosis using the THz radiation. For the delineation of the pathology margins, one usually resorts to collection and analysis of the THz images, which reflects spatial distribution of the tissue THz dielectric/optical properties over the sample aperture.
Applications of THz spectroscopy and imaging in medical diagnosis can be classified into three modalities depending on the diagnosis procedures:
● non-invasive methods do not violate the tissue integrity;
● minimally-invasive methods employ endoscopic or laparoscopic access to the internal tissues and organs;
● intraoperative methods resort of open access to internal tissues and organs during surgical treatment.
Relying on such a classification, in Fig. 1a-i, we showcase a few representative examples of the THz imaging for neoplasms of the larynx74, brain75, oral76, breast77, skin78, gastric79, liver80, colon81, and prostate82, respectively.
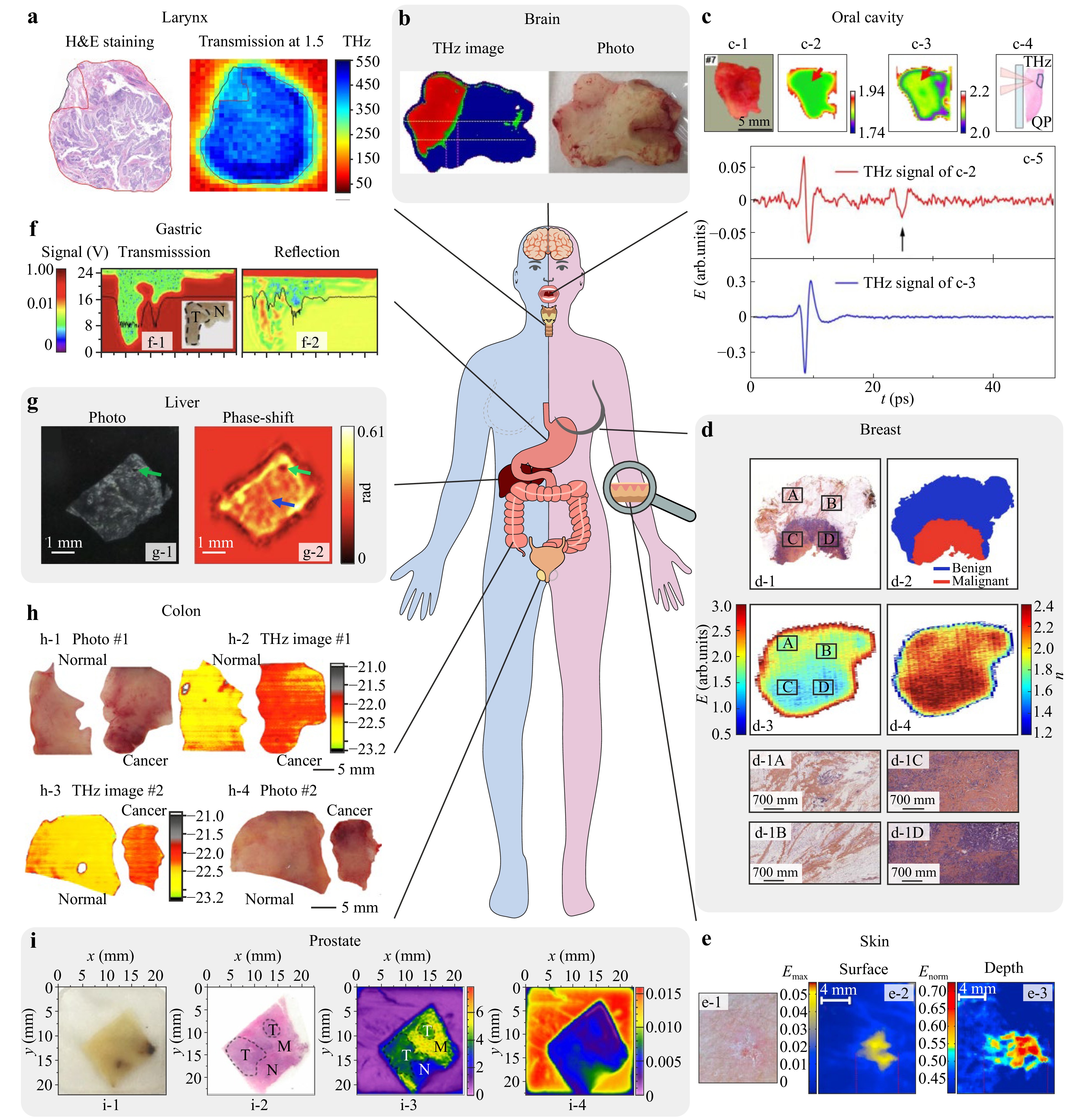
Fig. 1 A potential of THz technology in medical diagnosis of neoplasms. a THz image of laryngeal carcinoma ex vivo. Adapted from Ref. 74 published by Springer Nature under the CC BY 4.0 license. b THz imaging of the WHO grade 2 glioma ex vivo. Adapted from Ref. 75 published by Springer Nature under the CC BY 4.0 license. c THz spectra and images of the intact oral SCC. Adapted from Ref. 76 with the permission of Optica Publishing Group. d THz image, refractive index distribution (at 0.55 THz), and mapping of the sample of human breast carcinoma ex vivo. Adapted from Ref. 77 published by Springer Nature under the CC BY 4.0 license. e THz imaging of the skin BCC in vivo. Adapted from Ref. 78 with the permission of Oxford Academic. f Images at 0.58 THz of the paraffinized slice of human gastric adenocarcinoma (T stands for the tumor area) in transmission and reflection modes. Adapted from Ref. 79 published by IM Publications Open LLP under the CC BY 4.0 license. g In-line THz hologram of the human liver tissues with hepatocellular carcinoma. Adapted from Ref. 80 published by Springer Nature under the CC BY 4.0 license. h Cross-polarized THz images of the colon cancer ex vivo. Adapted from Ref. 81 published by SPIE under the CC BY 4.0 license. i. THz imaging of the paraffin-embedded prostate cancer. Adapted from Ref. 82 published by Optical Society of Korea under the CC BY 4.0 license.
-
Due to the limited depth of THz-wave penetration in tissues, the non-invasive diagnosis via the THz methods includes the study of the pathologies of the skin and mucosa83. In pilot articles69,70,78,84, different modalities of THz spectroscopy and imaging were applied for the differentiation between intact tissues and non-melanoma skin cancers ex vivo and in vivo, such as basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). In fact, these types of skin cancer were considered in pilot studies because, on the one hand, they occur frequently, and, on the other, they can be easily studied by existing laboratory THz instruments. The THz technology was shown to have potential in the pre- or intraoperative delineation of the margins of SCC and BCC, aimed at the gross-total resection of these cancers. For example, THz imaging can help to remove BCCs and SCCs during the Mohs micrographic surgery85. Fig. 1e shows the THz reflection-mode imaging of the skin BCC in vivo78, where panel e-1 shows a clinical photo, panels e-2 and e-3–THz parametric images revealing surface and depth characteristics of tissues.
In Refs. 86, 87 THz pulsed spectroscopy (aided by the consequent analysis of THz dielectric response of tissues) was found to be a prospective instrument for the non-invasive early screening of dysplastic nevi (moles) of the skin in vivo. Dysplastic nevi are precursors of melanoma (or the 0th stage of its development)88, while the latter is reportedly among the most dangerous cancers89. The capabilities of THz technology for the diagnosis of melanomas in situ were then confirmed in studies of both the melanomas from humans90 and formalin-fixed melanoma model from mice91. For pigmented nevi and melanomas, melanin affects the THz response of tissues and serves as an endogenous marker of pathology (along with tissue water), as shown in Ref. 92.
Oral tissue pathologies can also be diagnosed non-invasively using THz technologies. For example, in Ref. 93 THz pulsed imaging was used for the diagnosis of SCC in the freshly excised tongue ex vivo, while in Refs. 76, 94 a contrast in THz spectra and images between intact tissues and oral melanoma, SCC, and mucoepidermoid carcinoma was demonstrated involving both freshly excised and frozen tissue specimens ex vivo (Fig. 1c). The reflection-mode THz images of the surface of tissue specimen with oral cancer (SCC) obtained at -20° and 20°C (panels c-2 and c-3, respectively) have no significant differences, while THz waveform (panel c-4) from region indicated by the red arrow contains a second reflection pulse, which corresponds to the cancerous area that was located 1.3 mm from the surface76. It highlights the benefits of THz imaging at low temperatures, when parameters of frozen tissues have more pronounced differences.
The discussed THz measurements of the skin, mucosa, and tongue were carried out using tissue samples ex vivo, or, only in a few cases, tissues in vivo. Even when the pilot studies implied the tissues in vivo, the non-invasive THz diagnosis remains far from clinical trials and practice, which is predominantly due to the low ergonomics of the existing THz instruments. In fact, in the discussed measurements in vivo, the patients were oriented toward the cumbersome and clumsy THz systems, due to the absence of THz fibers, waveguides, and endoscopes capable of flexible delivery of THz waves to the aforementioned localizations. Thus, even non-invasive THz diagnosis demands THz endoscopic for operational convenience.
-
THz technologies can find their applications in the minimally-invasive diagnosis of neoplasms, with endoscopic or laparoscopic surgical access to tissues and internal organs. These techniques utilize natural orifices of human body or limit the size of incisions, which makes them less traumatic for the patient.
In this framework, THz technology holds strong potential in the diagnosis of cancers of the gastric, colon, and liver. Particularly, THz pulsed spectroscopy95 and imaging96 were used to distinguish between paraffin-embedded human gastric adenocarcinoma and intact gastric tissues. In Ref. 79 THz pulsed imaging was used for the diagnosis of the paraffinized human gastric adenocarcinoma at 0.58 THz in transmission and reflection geometries (Fig. 1f-1, f-2, respectively). Authors demonstrated sufficient contrast between intact gastric tissue and adenocarcinoma in the reflection-mode THz image.
Capabilities of THz microspectroscopy and in-line digital holography for the diagnosis of hepatocellular carcinoma in the human liver were demonstrated in Refs. 80, 97, respectively. THz imaging of liver tissue specimen post mortem was implemented to differentiate between regions of normal liver and metastatic liver cancer97. Fig. 1g shows photo and in-line THz digital hologram of the human liver tissues with hepatocellular carcinoma80. The green arrow indicates a vessel or damage caused by the freezing procedure, while the blue one points fibrosis of tissues.
THz pulsed imaging was also applied to differentiate cancer and intact tissues of the colon in the formalin fixed, paraffin embedded98, or freshly excised99 states. At the same time, in Ref. 81, the freshly excised colon specimens were visualized by the continuous-wave reflection-mode THz imaging system. In Fig. 1h, analysis of thus collected cross- and co-polarized THz images in logarithmic form (panels h-2 and h-3, respectively) uncovers the contrast between intact and cancerous tissues of the colon, which is consistent with histology.
All research on the minimally-invasive THz diagnosis of hard-to-access tissues and internal organs was performed using tissues ex vivo. In fact, despite the highlighted potential of THz technology, the absence of THz endoscopic systems makes it impossible to translate the developed principles to clinical practice. For such applications, the problem of THz endoscopy is especially challenging.
-
THz methods can also be applied for the intraoperative diagnosis of neoplasms in internal organs and tissues. Such diagnosis is performed during surgery with open access to the tissues and thus damages them.
In the context of intraoperative applications, THz pulsed spectroscopy and imaging were first considered as promising tools for delineating the margins of a breast tumor during surgery, the goal of which is the gross-total resection of a tumor with maximum preservation of intact tissues and minimum cosmetic damage from surgery100, 101. The ex vivo freshly-excised tissue specimens from humans were studied in this research, which demonstrated capabilities of THz technology in such a demanding branch of oncology. In Ref. 102, THz near-field microscopy was applied to study paraffin-embedded ductal carcinoma of the breast, aimed at improving the accuracy of tumor margin detection and studying the optical heterogeneities of tissues. During THz microscopy, the tissues were placed atop of the GaAs crystal, in which THz pulses were generated under the femtosecond laser excitation. In Ref. 103 intact and fat tissues, as well as cancer of the breast were differentiated using the multispectral THz reflection imaging aided by the immersion optical clearing of tissues. The immersion optical clearing allows for improving the penetration of THz waves in tissues and unmasking the non-water-related markers of pathology thanks to the substitution of tissue water (as a main THz-wave absorber) by a hyperosmotic agent (with much lower THz-wave absorption)104−106. For this, different agents can be applied to tissues, followed by the bimodal diffusion of an agent to tissues and water from them. In Fig. 1d, results of the THz pulsed imaging of freshly-excised human breast tissue ex vivo are reprinted from Ref. 77. Here, panels d-3, d-4 and d-2 correspond to the reflection-mode time-domain image, distribution of refractive index (at 0.55 THz) over the sample surface, and mapping of the tissue sample based on THz spectral data, respectively, while panels d-1A and d-1D are magnified areas of histological image (panel d-1) corresponding to A-D in d-1, d-3.
A vigorously-explored branch of THz application is the intraoperative neurodiagnosis27, 107. An ability to delineate margins of the glioblastoma (glioma of the WHO grade 4; WHO is the World Health Organization) model from mice and gliomas of the WHO grade 2–4 from humans using the THz reflectometry imaging ex vivo was uncovered in Ref. 75. In Fig. 1b, one of the pilot THz images of the WHO grade 2 glioma ex vivo is reproduced from Ref. 75, where the tumor area and margins are clearly determined by thresholding (as shown in red and green). In Ref. 108 THz pulsed spectroscopy was applied to retrieve the effective THz optical properties of the ex vivo gelatin-embedded intact brain tissues and the WHO grades 1–4 brain gliomas from humans, as well as to clearly demonstrate capabilities of THz technology for the discrimination between intact tissues and tumors. Then, in Ref. 73, these data were analyzed using the double-Debye and double-overdamped-oscillator models of complex dielectric permittivity, which made it possible to quantify the content and (to some extent) state of tissue water in the intact brain and gliomas. The Debye model remains the most widespread in THz biophotonics25, 51, while the one based on the overdamped oscillator is novel for this field, and it appears to be more physically rigorous73. In Refs. 64, 72, 109 the super-resolution THz solid immersion microscopy was applied to delineate margins of the freshly-excised glioma model 101.8 from rats ex vivo, visualize sub-wavelength ($ < \lambda $) heterogeneities of both intact tissues and a tumor of the brain (those can either complicate the detection of margins or give additional useful information), and uncover the THz birefringence of the Corpus Callosum of the brain. In Ref. 110, human brain gliomas of different grades were analyzed using the THz pulsed spectroscopy in the attenuated total internal reflection (TIR) mode. Substantial differences in THz response of tissues were observed not only between the intact tissues and tumors, but also between the isocitrate dehydrogenase (IDH) mutant and wild-type gliomas. Thus, THz technology can be useful not only in the detection of tumor margins, but also in the molecular diagnosis of IDH mutation (aimed at selecting the personalized treatment strategies).
In Ref. 111, differences between renal fibrosis and healthy kidney tissue from mice were observed in their THz pulsed images, while in Ref. 112, renal fibrosis from human was studied by the THz pulsed spectroscopy. In Ref. 113, an approach for differentiation between paraffin-embedded ovarian cancer and normal tissues using THz vibrational spectroscopy was proposed. In Ref. 114, THz pulsed spectroscopy and machine learning technique were applied together to differentiate between healthy prostate tissues and cancer of different grades according to the Gleason classification. At the same time, in Ref. 82, THz reflection-mode imaging of paraffin-embedded prostate cancer was performed, while the observed result are highlighted in Fig. 1i. A photo of representative tissue example is shown in panel i-1. It includes the tumor (T), normal prostate (N), and smooth muscle (M) tissues, as indicated in panel i-2. From panel i-3, one notices that the THz image based on the retrieved absorption coefficient shows sufficient contrast between intact tissues and a tumor, while the time-domain peak-to-peak THz data show less evident differences. Another example of the intraoperative application of THz imaging is associated with the detection of the laryngeal cancer margins74. In Fig. 1a, THz image of laryngeal carcinoma ex vivo is compared with the data of Hematoxylin and Eosin (H&E)-stained hystology. The tumor area is shown in red, while paracancerous tissue in black.
In addition to intraoperative studies of tissues ex vivo, THz imaging can aid classical histological microscopy, including its express modalities applied intraoperatively97, 115. An approach to improve the sensitivity of THz methods to changes in the effective optical constant of biological tissues and liquids involves the use of compact metasurface-based THz sensors, the resonant response of which changes pronouncedly in the presence of an analyte116, 117. Capabilities of such THz metamaterial sensors in diagnosis of BCC118 and melanoma119 of the skin, cancers of the gastric120, colon121, 122 and liver123, glioma of the brain124, Alzheimer's disease of the brain125 were demonstrated.
Despite such a wide variety of the intraoperative THz applications, they also remain far from clinics due to the absence of THz endoscopic systems capable of THz-wave delivery to hard-to-access tissues. Such systems should be ergonomic, as well as capable of combining with multimodal surgical tools and integration into the modern surgical workflows126.
-
A few groups proposed and implemented a straightforward approach to measure the THz dielectric response of a hard-to-access object, in which fiber-coupled THz PCA emitter and detector are located in close proximity to an object, while optical fibers are used for flexible delivery of the laser pump and probe beams to these PCAs.
-
Conventional free space THz pulsed spectrometers are cumbersome and can be applied mainly in a laboratory setting25, 127. This can be mitigated (to some extent) using the handheld THz probe based on a pair of fiber-coupled PCAs, an example of which is shown in Fig. 2a, b.
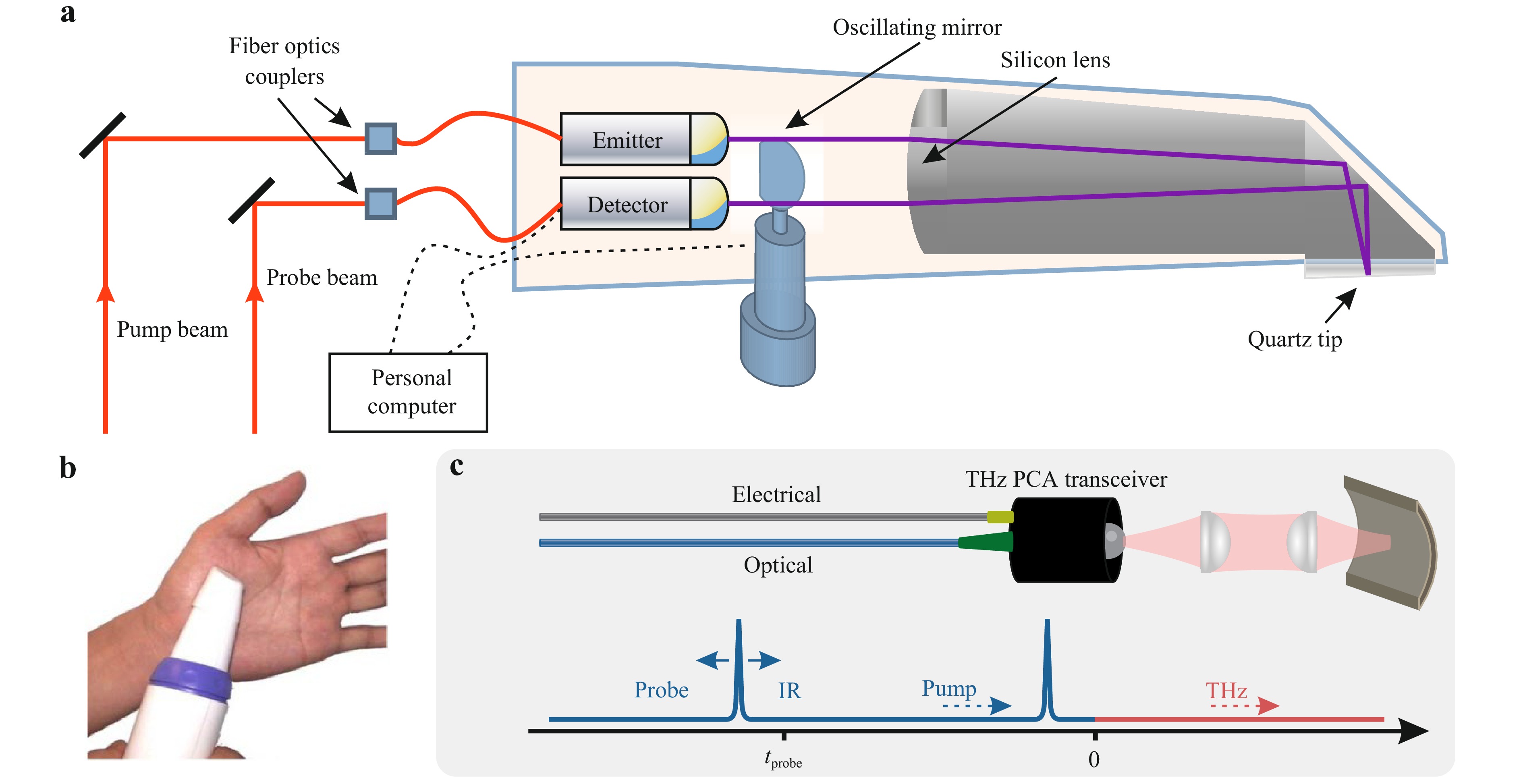
Fig. 2 THz endoscopic systems based on the fiber-coupled PCAs. a A schematic of the handheld THz endoscopic probe from TeraView Ltd., which is based on a pair of PCAs and an oscillating mirror. Adapted from Ref. 132 with the permission of Optica Publishing Group. b A photo of TeraView probe applied to study the human skin from the palm in vivo. Adapted from Ref. 133 with the permission of Optica Publishing Group. c A schematic of the fiber-coupled THz PCA transceiver, that uses variable time delay between the pump and probe pulses is shown schematically. Adapted from Ref. 135 with the permission of Optica Publishing Group.
Such systems operate in a similar manner to the conventional THz PCA-based pulsed spectrometer131−134. The near-IR femtosecond laser beam is split into the pump and probe sub-beams, which are guided through flexible optical fibers to the PCA emitter and detector, respectively. The dispersion of femtosecond laser pulse in fibers is compensated using a judiciously-designed compensator based on diffraction gratings or other principles. The delay between the pump and probe beams is varied using a mechanical translation stage (or other approaches) allowing the registration of the time-varying THz electric field. The emitted THz pulse passes through an optical system in order to be focused on a sample and then then the reflected THz signal is collimated and directed toward the detector. The beam can also be deflected by an oscillating mirror (Fig. 2a)128, a pair of rotating optical wedges (the so-called silicon Risley Prism Scanner129, 135), or other systems, which makes it possible to raster scan of an object by the focused THz beam for 1D or 2D imaging. A reference THz window is commonly used to position an object in the focal plane of the THz beam, so that the THz signal reflected from the window-object system is analyzed.
Such handheld THz endoscopes were used to study the THz spectra and images of superficial human tissues128, 129, 136−139. Despite the significant potential in medical spectroscopy and imaging, the energy efficiency of such systems is much smaller than that of the common THz pulsed setups. Therefore, such systems are commonly aided by the advanced data processing techniques (for example, the frequency-wavelet domain deconvolution129, 140) to improve their performance, signal-to-noise ratio, spectral range and resolution. Nevertheless, even with the advanced data processing routine, the spectral range of such THz endoscopic systems remains much narrower (< 2 THz) as compared to the common THz pulsed systems (< 4-5 THz) due to the reflection-mode arrangement and the additional Fresnel loss of the THz beam power.
In Ref. 128 the handheld THz endoscopic system was developed to raster scan a sample (with the field of view of 15 × 2 mm2 and the spatial resolution of $ \simeq 1 $ mm) by the oscillating mirror (Fig. 2a). It was applied to improve the accuracy of the intraoperative assessment of the human breast tumor margins. Quite similar THz endoscopes based on a pair of the fiber-coupled PCAs were reported in Refs. 137−139. Most recently, photonic integrated circuits were applied to deliver the laser pump and probe beams to the PCA emitter and detector, respectively141. In fact, the principles of integrated photonics should open the way for creation of portable THz pulsed systems, which by now are still far from applications.
Finally, we notice that for the considered systems, special attention still needs to be paid to the data processing and the THz dielectric response quantification137, 142. Until now, they are very rare and expensive. It would take considerable time, research, and engineering efforts to bring them closer to clinical spectroscopy and imaging.
-
An attractive arrangement of the fiber-coupled PCA-based THz endoscope relies on compact THz PCA transceiver modules, also coupled to optical fibers. Concepts of such a transceiver were introduced based on twin dipole PCAs fabricated on a single chip143, or a single PCA that serves simultaneously as an emitter and a detector144, 145. They were designed mostly for the near-field sensing applications. In Ref. 130, PCA transceiver was applied in THz remote measurements (Fig. 2c). Although the observed results of THz imaging and thickness measurements demonstrated the potential of this technique, the developed system suffered from a narrow bandwidth ($ \nu_{\rm{max}} < 0.5 $ THz) and a low signal-to-noise ratio. Further investigations are essential to make these systems practically available.
Finally, we note the THz near-field imaging modalities based on THz PCA cantilevers. The PCA-based near-field probes can be placed in close proximity to an object to collect the THz near-field data. In Refs. 146, 147, the spatial resolution of such systems was as high as ~ 1 μm. This allows one to visualize separate (but quite large) cells in biological tissues. Meanwhile, the problems of THz data processing and quantification of the THz optical constant of an object are still challenging for such THz near-field imaging modalities.
-
We now discuss the potential of classical THz endoscopy, the enabling technology of which is the flexible THz fiber, or the rigid THz waveguide. Such THz fibers/waveguides operate using different guiding mechanisms, material platforms, and fabrication strategies148−150. For applications in medical endoscopy, the THz fibers should meet the following demands:
● They should be able to guide THz waves over considerable distances (~ 1-1 m) with relatively low loss ($ \leq1 $-10 dB/m) and dispersion (< 1 ps (THz cm−1)). This would make it possible to deliver THz radiation from an emitter to hard-to-access tissues and internal organs (placed in contact with the output end of the probe), detect the reflected and back-propagated THz signal, and then quantify the THz dielectric response of tissues.
● They should meet the demands of minimally invasive laparoscopy and endoscopy, which require a fairly small outer diameter (< 3-5 mm) and (preferably) flexibility of the endoscope. Considering the quite small endogenous contrast (~ 1-5%) between intact tissues and pathology in the THz spectra and images, the sources of noise such us bending and shrinkage of the THz fibers/waveguides (owing to the mechanical loads) should be engineered to have a minimal effect on the fiber guiding properties and the measured THz data.
● The THz fiber/waveguide materials must be inert to biological tissues and liquids. In order to make possible multiple use, they also must be resistant to the medical sterilization conditions, such as the temperature up to 190℃, the pressure up to 2 Bar, and the chemicals like ethylene oxide (or alternative sterilization methods151).
In fact, simultaneous achievement of all these characteristics is quite a daunting task. To date, only a few fiber/waveguide-based THz endoscopes were realized and applied to study of biological objects.
-
We now review and classify the existing THz fibers/waveguides by their corresponding guiding mechanism25, 57, 58, and discuss their potential in THz endoscopy.
-
Waveguides with the guided mode confinement between metal plates or inside a hollow metal tube (those are well-known in the microwave range152) were translated to the THz gap a decades ago153, 154. In such structures, THz waveguiding results from reflection of waves from the metal walls, while the guided mode is confined in a hollow core. When the inner diameter of such a waveguide is larger than the wavelength, the propagation loss can be low, but low-loss frequency band is limited. However, when the wavelength becomes comparable with the diameter and a waveguide operates close to the cutoff frequency, the propagation loss and modal dispersion increase significantly. Moreover, at higher frequencies, such waveguides suffer from the additional conducting losses of radiation and from multimode operation regimes, with the resultant intermodal dispersion. In vast majority of cases, they are non-flexible, with a few exceptions, such as the THz waveguide in the form of a hollow glass (Fig. 3a)155 or polymer165 tubes with inner metal (or, complex polymer-metal) coating.
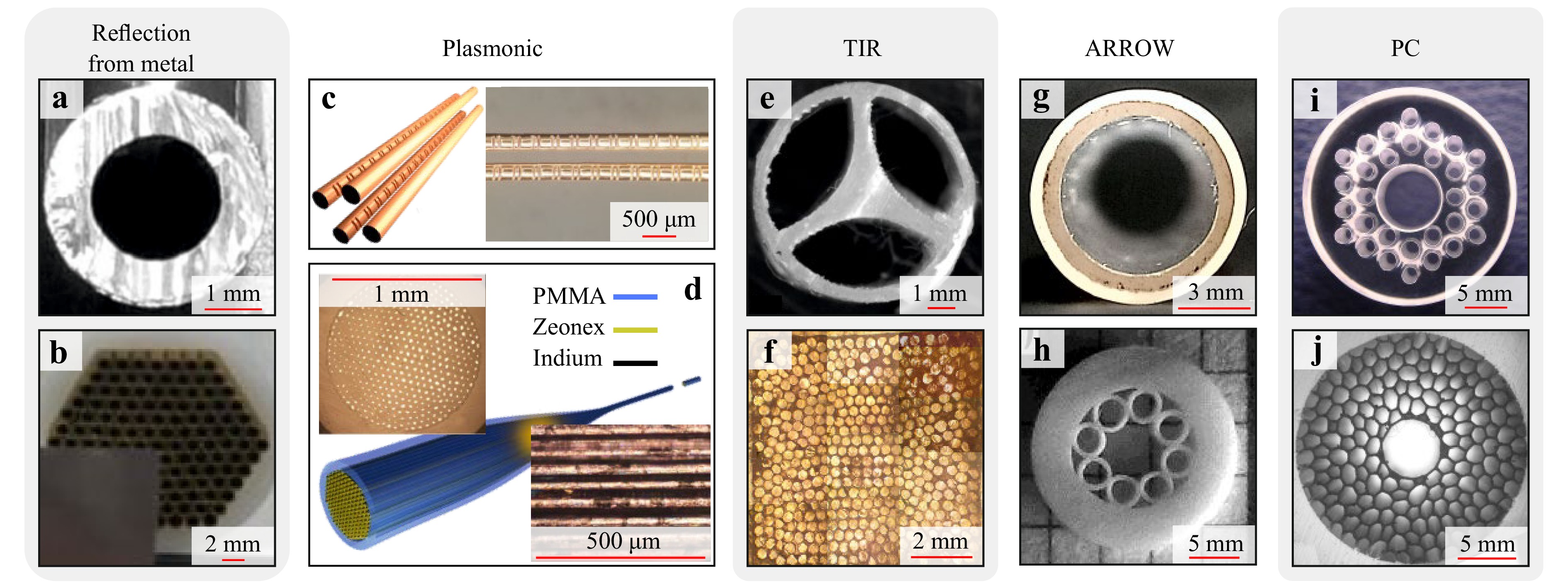
Fig. 3 Representative examples of the THz waveguides and fibers. a Hollow-core polystyrene-Ag-glass waveguide. Adapted from Ref. 152 with the permission of AIP. b Bundle of the hollow-core Cu THz waveguides. Adapted from Ref. 153. with the permission of ACS. c Four-wire plasmonic waveguide with the Bragg gratings engraved along the two of them. Adapted from Ref. 154 published by Springer Nature under the CC BY 4.0 license. d Plasmonic bundle of metal wires suspended in a dielectric and fabricated via the polymer drawing. Adapted from Ref. 155 published by Springer Nature under the CC BY 4.0 license. e 3D printed microstructured polymer step-index TIR fiber with a subwavelength core suspended in free space by three thin bridges in the middle of an encapsulation tube. Adapted from Ref. 156 published by Springer Nature under the CC BY 4.0 license. f. Tapered bundle of the high-refractive-index EFG-grown sapphire step-index TIR fibers designed for the super-resolution THz imaging. Adapted from Ref. 157 with the permission of APS. g Hollow-core ARROW waveguide based on the EFG-grown polymer-coated sapphire tube. Adapted from Ref. 158 with the permission of AIP. h Revolver-like microstructured polymer ARROW waveguide. Adapted from Ref. 159 published by EDP Sciences under the CC BY 4.0 license. i EFG-grown sapphire microstructured PC waveguide. Adapted from Ref. 160 with the permission of Wiley. j. 3D printed microstructured hyperuniform polymer PC waveguide. Adapted from Ref. 161 with the permission of Wiley.
Due to their thermal conductivity and resistance, hollow metal THz waveguides were successfully used for the power delivery into cryogenic environments166, 167. In turn, owing to the low chemical inertness of metals, such waveguides have a limited potential in chemical sensing and medical applications. Metal tubes can be assembled in a bundle for the THz imaging applications (Fig. 3b)156. To further improve the spatial resolution of such system and even overcome the Abbe diffraction limit, one can resort from ordinary metal tubes to the metal-coated high-refractive index sapphire fibers, produced by the edge-defined film-fed growth (EFG) technique168, 169 and arranged in a bundle170. High THz refractive index of sapphire leads to a stronger confinement of a guided mode in the core of each individual fiber. This turns into the advanced resolution—as high as 0.3–0.5$ \lambda $. At the same time, such optical fiber bundles suffer from increased dispersion and propagation loss as a result of the guided mode-sapphire core interaction.
-
Plasmonic waveguides consist of a single metal wire or ribbon, or an array of such wires or ribbons (Fig. 3c, d). It is possible to excite the plasmonic mode on the surface of a conductor and transport the THz pulses with virtually no dispersion and low losses. For the first time, THz plasmonic waveguidance along a subwavelength single metal wire was reported in Ref. 171. Significant disadvantages of the one-wire transmission, such as low coupling efficiency of linearly polarized field172 and high bending losses173, restrain their practical utility. In turn, the two-wire plasmonic scheme can be excited by a linearly polarized field, supports the propagation of broadband THz waves (including the strong THz fields174) with low loss and low dispersion, and appears to be resistive to bending loss175, 176.
The problems of plasmonic waveguide handling and guided mode scattering due to interactions with external obstacles also limit their utility. To mitigate these problems, plasmonic waveguides with a porous dielectric support were introduced175, 177. Nevertheless, interactions between a guided mode and a dielectric support material increase the dispersion and propagation loss of such waveguides, and thus loose their attractive guiding properties. Here, we also mention plasmonic waveguides with dielectric gratings178−180 or patterned metal surface (Fig. 3c)157 designed for THz sensing.
In the visible and IR ranges plasmonic modes are tightly confined near the metal wire/ribbon, while their dimensions are as small as $ \lambda / 10 $-$ \lambda /100 $ and for two-wire waveguide configuration the plasmonic modes can be confined to sub-lambda sizes even for THz range. In this way, an individual plasmonic waveguide (as probe in near-field THz microscopy) and a wire medium (a regular array of plasmonic waveguides those transfer the THz image from the object to the imaging plane)181 have a strong potential in the THz microscopy with the resolution far beyond the Abbe limit (Fig. 3d)158, 182, 183. However, the practical utility of such super-resolution THz imaging modalities is still doubtful.
-
Step-index dielectric fibers operating using the TIR guiding mechanism and featuring the higher-refractive-index core and lower refractive index cladding, which ensures, were also implemented in the THz range. Such fibers can possess a bulk (polymer or crystalline)184−186 or a porous187−189 core. The core can be suspended in free space or coated by a low refractive index cladding in order to prevent de-coupling of guided mode due to the evanescent field interactions with external obstacles159, 190. Fig. 3e shows another approach to prevent such de-coupling, in which the sub-wavelength core is suspended in free space and handled in the middle of an encapsulation tube by thin dielectric bridges159. Step-index fibers based on low-losses polymers offer low-to-moderate propagation loss (< 1 dB/m; it is enough to guide THz waves over the distances of ~ 1 m) while often showing high dispersion, attributed to the confinement of a guided mode in a core material, as well as the high dielectric contrast between a core and a cladding185, 191.
Step-index dielectric fibers have potential in high-resolution THz imaging. The flexible fibers, based on the polyethylene192, cyclic olefin copolymer193 and EFG-grown sapphire194, 186 were used as the probes in the THz scanning-probe near-field optical microscopy. Particularly, the sapphire fiber-based THz microscope with the resolution of $ \simeq0.25 \lambda $ was used for the pilot experimental observation of a photonic hook effect195, 194. Similar THz microscopes based on the flexible step-index optical fibers were used in the THz imaging of the cancers of the liver196 and the breast197, 198. Another option of the THz super-resolution imaging was suggested in Ref. 160, where high-refractive-index EFG-grown sapphire optical fibers were arranged in a tapered bundle (Fig. 3f). Thanks to a strongly sub-wavelength confinement of a guided mode in each individual fiber, such a bundle is capable of near-field THz imaging with the resolution down to $ 0.3\lambda $.
Nevertheless, practical implementations of the step-index THz fibers is still challenging due to the absence of appropriate cladding materials56 and their low-to-moderate optical performance. The lack of cladding makes the use of such fibers in THz endoscopy suboptimal.
-
The antiresonant reflecting optical waveguides (ARROW) with their complex cladding structure199 are quite common in the THz range. In ARROW waveguides, a guided mode is localized in a hollow core because of the reflections from a layered or microstructured cladding. These reflections are due to the Fabry-Perot antiresonant conditions in a cladding for the radial component of a wave vector200. In this case, the energy coupling between the core-guiding and lossy cladding modes does not occur, and the propagation loss is reduced. Inversely in the resonant case, strong coupling between the core-guided and lossy cladding modes takes place, which leads to the strong propagation loss. The spectral bands of low and high absorption associated with the antiresonance and resonance conditions, respectively, usually alternate in the fiber loss spectra.
Even a simple dielectric tube can operate as an ARROW waveguide (Fig. 3g)201, but waveguides of more complex cladding structure are reportedly more efficient202. Typically, a cladding has the form of a regular layered structure with refractive index modulation. In Refs. 162, 203−206, different types of ARROW structures were studied to reduce the propagation and bending losses, such as the revolver-like polymer ARROW waveguide obtained in Refs. 162, 203, 207. (Fig. 3h). A favorable combination of the large transmission band, overall low dispersion and propagation loss (~ 10−1 dB/m) gives ARROW waveguides many advantages. Their disadvantages include high modal dispersion near the Fabry-Perot resonances and (what is even more important for endoscopy) large diameter of such waveguides requires by the ARROW guiding mechanism ($ \sim 10 \lambda $). Strong intermodal interference and dispersion153, 163 also limit practical utility of the ARROW THz waveguides.
-
In the photonic crystal (PC) waveguides, a mode is confined in a hollow or solid core due to the coherent reflection of waves from a complex periodic cladding. Cladding periodicity leads to the formation of a spectral region called Photonic Band Gap (PBG) within which extended photonic states are suppressed in the cladding. From the practical point of view it means that periodic cladding becomes highly reflective, thus capable of confining light in the hollow core. Cladding can be formed in a different manner, including the use of a periodic array of hollow channels163 (Fig. 3i), axisymmetric periodic multilayers208, or even hyperuniform disordered reflectors164 (panel j).
PC waveguides can deliver radiation of common gaussian-like linearly polarized THz-wave sources208−210. The simplest axisymmetric cladding can be formed by an array of concentric layers with different refractive indices, such as sequences of polymer and free space or two distinct polymers. PC waveguides with free space in their structure require dielectric supports187, 191, 211. PC waveguides with a 2D lattice of hollow channels are also commonly used, where different types of 2D crystalline lattices and geometries are used210, 212−214.
The guiding properties, material platforms, and strategies applied for the manufacturing of ARROW and PC waveguides are generally similar. PC waveguides tend to have large diameters ($ \sim 10\; \lambda $), often larger than those of the ARROW waveguides. Fibers/waveguides with a wide variety of cross sections can be manufactured of different polymers (PE, polypropylene, cyclic olefin copolymer (TOPAS), Teflon, etc.) using 3D printing164, 204−206, 208, stacking and drawing203, drilling and drawing215 and other techniques. Moreover, EFG method of shaped crystal growth can be applied to produce ARROW and PC waveguides of sapphire161, 163, 201, 216, 217, with its advanced thermal and radiation strength, chemical inertness and biocompatibility168, 169. Such sapphire waveguides are capable of solving numerous problems in THz biomedicine.
-
To date, only a few THz endoscopes (mainly based on dielectric or metal tubes) have been applied to characterize hard-to-access objects192, 218−220. In Ref. 218, a single-channel THz endoscope was developed and applied for THz imaging in transmission and reflection modes, where an object was raster scanned by the output endoscope end (Fig. 4a-d). In particular, it was applied for the THz imaging of human colon cancer. The THz waveguide applied in this endoscope has a small outer diameter (1-2 mm), which makes it suitable for integration into conventional endoscopic tools and applications in minimally-invasive THz diagnostics. In Ref. 219, similar THz endoscope was developed relying on a Teflone tube, served as both a THz waveguide and a scanning probe (Fig. 4e, f). It was also applied for the remote THz sensing of ammonium-chloride aerosols produced by the chemical reaction between hydrochloric acid and ammonia vapors. This system is compact and, thus, can be applied in the industrial or biomedical THz endoscopy. Finally, in Ref. 220, remote spectroscopy of theophylline was performed using by the THz endoscope made of a flexible polycarbonate tube with the inner silver coating and the inner diameter of 3 mm. In Fig. 4g-h, thus measured THz absorption is reproduced, where the absorption peaks of theophylline are highlighted by the yellow-shaded area. Most of the existing THz endoscopes focus on obtaining qualitative information about an object, or sensing applications. Meanwhile, quantification of the THz dielectric response of an object, which is so needed in biomedical applications, remains poorly addressed.
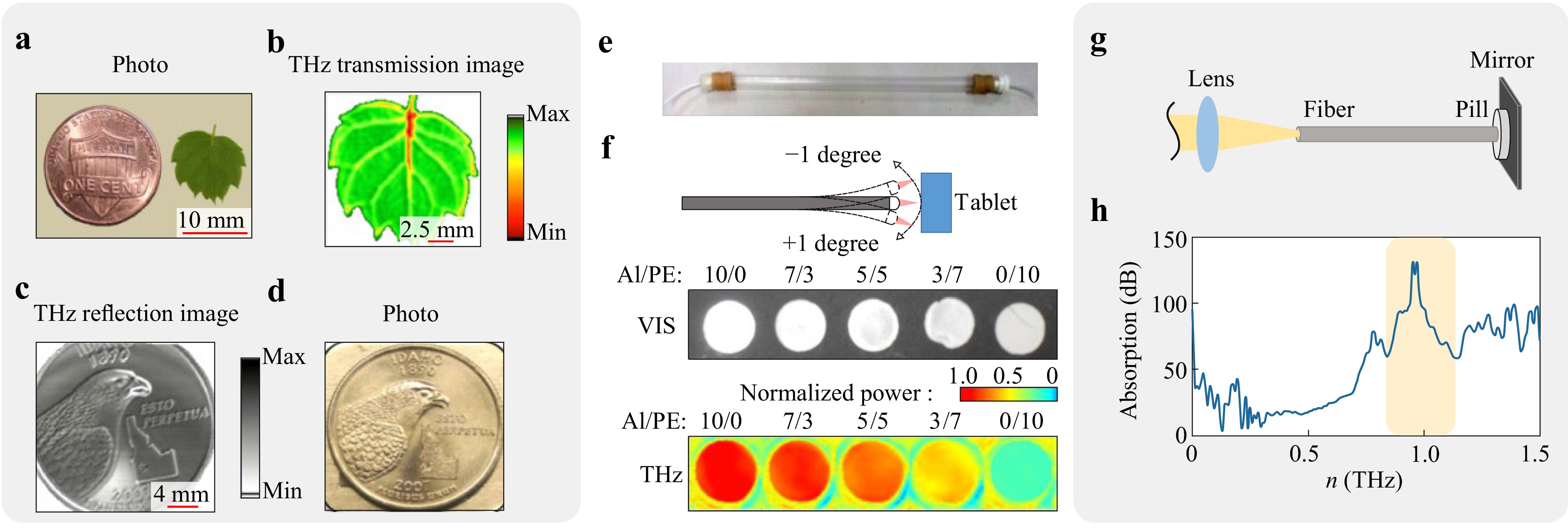
Fig. 4 Qualitative THz endoscopy of hard-to-access objects. a-d Transmission- and reflection-mode THz imaging of different objects using a single-channel endoscope based on the flexible metal-coated THz waveguide. Adapted from Ref. 218 published by SPIE under the CC BY 4.0 license. e Teflon pipe waveguide with the inner diameter of 8 mm, and wall thickness of the 1 mm, which is used for sensing of the chemical reaction. f Waveguide-based reflection-mode THz image to test tablets with the different fractions of Al and PE powders. Panels e and f are adapted from Ref. 219 with the permission of Optica Publishing Group. g, h Schematics and results, respectively, of the waveguide-based reflection mode spectroscopy of the theophyllyne pill. Adapted from Ref. 220 with the permission of Optica Publishing Group.
Most recently, in Ref. 161, quantitative calculations of the optical constants from the THz endoscopic data have been performed. For this, the ARROW THz waveguide was fabricated using the EFG-grown sapphire tube with a Teflon coating (Fig. 3g). From the object’s side, it was closed by a flat $ \simeq 1.0 $-mm-thick sapphire window that serves as a Fabry–Perot sensor of the complex refractive index. It is worth noting that similar approach to quantify the THz optical constant of an analyte handled at the shadow side of a reference window was earlier used in the reflection-mode (non-endoscopic) THz spectroscopy based on backward-wave oscillators (BWOs)221. In Ref. 161, the THz endoscope was also attached to the reflection-mode BWO spectrometer (Fig. 5a). By measuring the THz reflectivity (by power) of the waveguide with a sample in contact with the output window, and analyzing the Fabry-Perot resonances in the observed THz spectra, both the THz refractive index $ n $ and absorption coefficient $ \alpha $ of an analyte were retrieved.
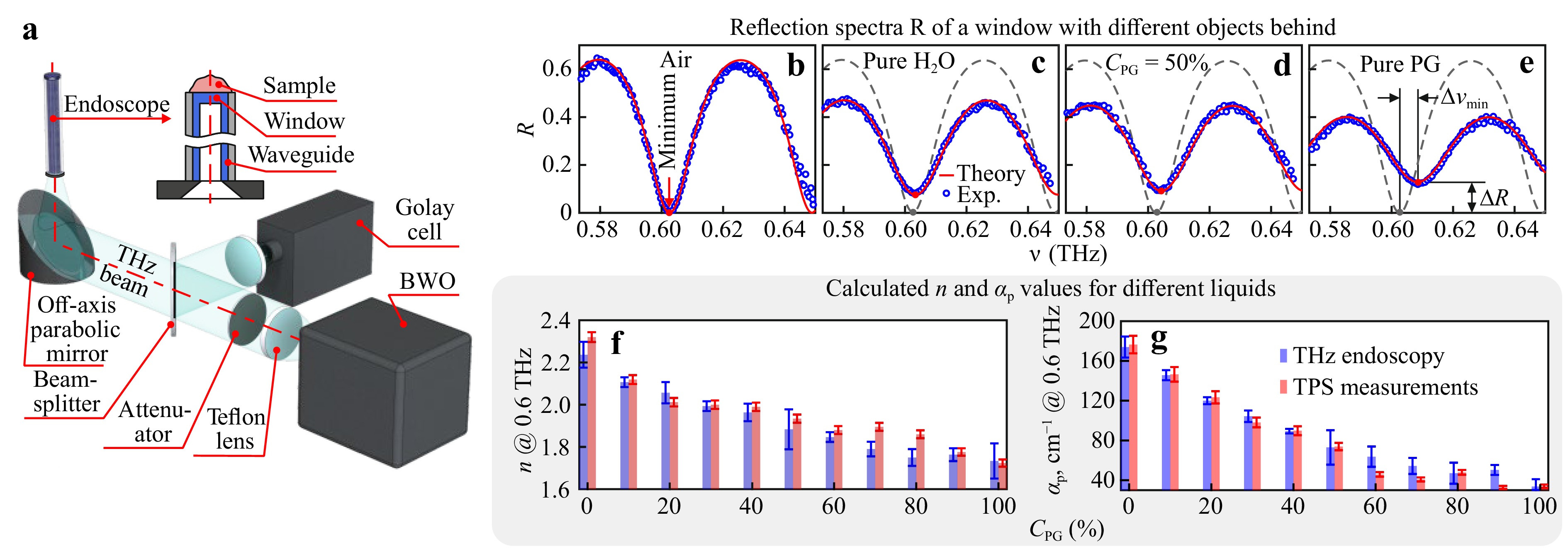
Fig. 5 Endoscopic measurements of the THz optical constants161. a Schematic of the THz endoscopic system that uses the EFG-grown Teflone-coated sapphire waveguide and the reflection-mode BWO spectrometer. The output end of a waveguide is closed by a $ \simeq 1.0 $-mm-thick sapphire window, which is in contact with an analyte and which serves as a Fabry-Perot sensor of complex refractive index. b-e Reflection spectra $ R $ (by power) of the sapphire window, which is in contact with air, pure water, 50% aqueous solutions of PG (by volume), and pure PG, respectively. Changes in the amplitude $ R_{{\rm{min}}} $ and spectral position $ \nu_{\rm{min}} $ of the Fabry-Perot minimum near $ \nu = 0.6 $ THz are analyzed to quantify $ n $ and $ \alpha $-values. f, g Endoscopic data on the THz refractive index $ n $ and power absorption coefficient $ \alpha_{\rm{p}} $ at $ \nu = 0.6 $ THz calculated for PG aqueous solutions with the volume fractions in the CPG = 0–100% range, and compared to the THz pulsed spectroscopy data. All panels are adapted from Ref. 161 with the permission of AIP.
Complex refractive index of an analyte was estimated from the amplitude $ R_{{\rm{min}}} $ and spectral position $ \nu_{{\rm{min}}} $ of the Fabry-Perot reflectivity minimum222:
$$ \begin{split} R_{{\rm{min}}} &= \left| \frac{ \widetilde{r}_{12} - \widetilde{r}_{23}}{ 1 - \widetilde{r}_{12} \widetilde{r}_{23}} \right|^2 ,\\ \nu_{{\rm{min}}}&= \frac{c_{0}}{4 d n_{2}'} \left( \frac{\varphi}{\text π} + (2m+1) \right) \end{split} $$ (2) where $ \widetilde{r}_{12} $, $ \widetilde{r}_{23} $, and $ \varphi $ are the Fresnel reflection coefficients (by field) and wave phase shift, respectively:
$$ \begin{split} \widetilde{r}_{12} &= \frac{ \widetilde{n}_{{\rm{mode}}} - n_{2}'}{ \widetilde{n}_{{\rm{mode}}} + n_{2}'}, \\ \widetilde{r}_{23} &= \frac{ n_{2}'-\widetilde{n}}{n_{2}'+\widetilde{n}},\\ \varphi &= \arctan \left(\frac{2 n_{2}' n''}{ n_{2}'^2 - n'^2 - n''^2}\right) \end{split} $$ (3) $ d $ is the sapphire window thickness, $ m=0, \pm 1, \pm 2 $ is an integer, $ \widetilde{n}_{{\rm{mode}}} $, $ n_{\rm{2}}' $, and $ \widetilde{n}=n'- i n'' $ are the refractive indices of the guided mode (in our case, it is close to unity), sapphire windows, and analyte, respectively. The details of the THz optical properties estimation can be found in Ref. 161.
In Fig. 5b-e, examples of the Fabry-Perot resonances in reflection spectra $ R $ (by power) are shown for free space and different liquids placed in contact with the output end of the endoscope. As test liquids, deionized water and aqueous solution of propylene glycol (PG) were applied. In panels f and g, the quantified THz refractive index $ n $ and power absorption coefficient $ \alpha_{\rm{p}} $ at $ \nu = 0.6 $ THz are shown for different aqueous solutions of PG. The estimated THz optical constants agree well with the THz pulsed spectroscopy data, which justifies the high fidelity of the developed method.
Clearly, more and more attention is paid to THz endoscopy, which is driven by emerging applications of THz technology in medical diagnosis, industrial quality control, and non-destructive testing. There is no doubt that applied research and engineering efforts in the area of THz fiber optics and endoscopy will make it possible to develop THz systems capable of addressing modern problems in various branches of science and technology.
-
Today, THz technology is still used mainly for the ex vivo investigations of tissue specimens, or the in vivo measurements of easily-accessible tissues and organs, such as the skin60. It stands in line with other emerging diagnostic tools, with its set of advantages and limitations. In this regard, THz endoscopy can become an efficient auxiliary technique in surgical workflow. The significant reason for this is the label-free character of the observed contrast between intact tissues and pathologies in the THz range25, 27, 59. Moreover, recent research demonstrate the safety of low-power THz beams applied in common THz pulsed systems51.
The overviewed developments in the field of THz waveguiding allow to make a step forward towards THz wave delivering to hard-to-access tissues and internal organs. The results of the past decade show us the appearance of innovative material platforms and strategies for the THz waveguide fabrication, as well as the improvement in the optical performance of such waveguides (including reduction of their dispersion, propagation and bending losses, increase in the stability and quality of their cross section over a considerable length improvement of their flexibility, chemical inertness, and biocompatibility). The use of different materials and guiding mechanisms in a single waveguide makes it possible to tune the waveguide properties within wide limits to accommodate the needs of THz applications25,57, 169. The first prototypes of THz endoscopic systems appeared recently, showcasing a variety of novel applications of this technology. Their pilot studies show their potential, as well as uncover their problems and drawbacks. Together with the improvement of innovative ergonomic room-temperature THz emitters and detectors223−227, the reviewed technologies form the necessary base for designing the THz medical and industrial systems.
-
In this review, we considered recent efforts addressing a challenging problem of the THz endoscopic measurements of hard-to-access objects. We briefly overviewed the THz applications in diagnosis of neoplasms with different nosologies and localizations. We considered the two existing principles of THz endoscopy. The first relies on the THz PCA technologies, which enable the THz-wave generation and detection in close proximity to a hard-to-access object, while optical fibers are used flexible delivery of a laser radiation to PCAs. The second technology one uses THz fibers/waveguides for delivering the THz waves to an analyte and then detecting the reflected and back-propagated THz signal. Remarkable examples of different THz endoscopic systems are discussed.
-
The work of G.M.K., S.V.G., and K.I.Z. on most parts of this review was supported by the Russian Science Foundation, Project #25-79-30006, while the work of D.S.P. on Sec. 3, was supported by the state assignment of the NRC “Kurchatov Institute”
Terahertz endoscopy of hard-to-access objects in the context of neoplasms diagnosis–A review
- Light: Advanced Manufacturing , Article number: (2025)
- Received: 04 February 2025
- Revised: 03 July 2025
- Accepted: 09 July 2025 Published online: 21 August 2025
doi: https://doi.org/10.37188/lam.2025.058
Abstract: Although terahertz (THz) spectroscopy and imaging offer a variety of applications in medical diagnosis of malignant and benign neoplasms, their translation into clinical practice is hampered by the absence of endoscopic systems capable of sensing the THz optical properties of the hard-to-access tissues. In this review, we focus on recent attempts to address this challenge. To better highlight the need for THz endoscopes, we start with a brief overview of THz medical applications, with an emphasis on neoplasms diagnosis. We then consider the two existing principles of THz endoscopy. The first uses the fiber-coupled THz photoconductive antennas (PCAs) for the THz generation and detection in close proximity to a hard-to-access object, where optical fibers are applied to flexibly deliver the laser pump and probe beams to the THz emitter and detector. The key technology of the second approach is the THz optical fibers capable of delivering the THz waves to an analyte and then detecting the reflected and back-propagated THz signal. Despite this approach still lacking the efficient commercially available THz fiber optics, most recent developments pave the way to solve these problems. In this review, several notable examples of THz endoscopic systems based on different guiding mechanisms, material platform, and manufacturing strategies are discussed.
Research Summary
Terahertz endoscopy of hard-to-access objects in the context of neoplasms diagnosis
Translation of terahertz (THz) technology to clinical practice is hampered by the absence of endoscopic systems capable of THz-wave delivery to hard-to-access biological tissues. In this review, recent attempts to address this problem are considered. G.M. Katyba and co-authors start with a brief overview of THz medical applications, with an emphasis on neoplasms diagnosis. Then, the two existing principles of THz endoscopy are considered. The first uses the fiber-coupled THz photoconductive antennas for the THz generation and detection in close proximity to a hard-to-access object. The second is based on the THz optical fibers capable of delivering the THz waves to the object and then detecting the reflected and back-propagated THz signal. In this review, several notable examples of THz endoscopic systems with different guiding mechanisms, material platform, and manufacturing strategies are also discussed.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article′s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article′s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.






 DownLoad:
DownLoad: