-
The development of scintillators capable of efficiently converting X-ray photons into visible light is essential in advancing radiation detection for medical diagnostics, security screening, and industrial imaging1,2. In the pursuit of scintillators with balanced overall performance (manufacturing costs, long-term stability, radiation conversion capacity, etc.), full-inorganic metal halides have proven to be promising alternatives. As a typical representative, lead-based metal halide scintillators, such as CsPbBr3, have demonstrated superior scintillation properties, including exceptional radiation absorption, highly efficient photon conversion, and excellent light output3,4. Experimental evidence has demonstrated that strategies such as cation doping, alloying, and halide substitution can effectively modify electronic band structures, thereby significantly modulating their exciton dynamics and emission wavelengths. However, the toxicity of lead has limited its environmental safety, spurring interest in safer and more sustainable alternatives5. The exploration of lead-free metal halides with lower toxicity has been a hot topic in recent years. To date, major attention has been paid to Zr-, Cu-, Zn-, In-, and Ag-based metal halides such as Cs2ZrCl66,7, Rb2CuBr38, Cs2Cu3I59,10, Cs2ZnBr411, Cs2AgInCl612,13, CsAg2I314, and Rb2AgCl315. Through rational structural design and impurity doping, these low-dimensional lead-free metal halides are considered potential candidates for next-generation scintillators16,17.
Owing to the rich 4f-electron energy levels and high atomic numbers of lanthanide (Ln) ions18, doping inorganic metal halides with Ln ions not only allows precise tuning of their optical properties but also enhances their X-ray attenuation capability. Accordingly, many potential Ln-doped metal halides, such as Cs2AgInCl6:Yb19, CsPbBr3:Eu20, and CsMnBr3:Yb21, have been reported over the last five years. The doping strategy relies on adding a certain amount of Ln3+ ions to metal halide hosts, where doping concentrations that are too high or too low may induce a non-uniform dopant distribution and inadequate performance improvement. As a promising alternative, Ln-based metal halide scintillators built entirely on luminescent Ln3+ ions can guarantee a uniform distribution and high density of active centres, giving rise to a more efficient energy conversion and a higher light yield. Nevertheless, Ln-based metal halide scintillators initially did not receive as much attention as the aforementioned Ln-doped candidates, mainly because of their synthetic challenges in solution-based mass production. Traditional methods often require high temperatures and elaborate processing conditions, which, along with the problems of low product yield and quality, restrict large-scale application22.
In a newly published paper in Light: Science & Applications, Li et al. reported a novel, rapid, and mild recrystallisation method to prepare A3BX6-type Ln-based metal halides with good photoluminescence and scintillation properties23. Utilizing a facile solvent-antisolvent strategy involving the successive addition of methanol and ethanol, this recrystallisation approach significantly streamlines the rapid synthesis of a wide array of Ln-based metal halides (Cs3LnCl6; Ln = Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, or Lu) under ambient conditions. This method not only yields highly crystalline Ln-based metal halides but also supports the large-scale production and recyclable recrystallisation of materials, which is a critical step toward sustainable material processing. Moreover, this method enabled the preparation of high-entropy multi-element crystals (e.g. Cs3(TbDyHoErTm)Cl6), which were previously achievable only at high temperatures (>900 °C)24,25.
Li et al. demonstrated that the synthesised Cs3LnCl6 crystals share a monoclinic C2/c crystal structure, forming a zero-dimensional (0D) framework where individual [LnCl6]3− octahedra are isolated by Cs+ ions (Fig. 1a). As the ionic radii of Ln3+ ions range from 1.01 (Ce3+) to 0.86 (Lu3+) for fixed octahedral coordination26, the identical crystal phase of Cs3LnCl6 crystals implies that they indeed have great structural tolerance. The 0D structural arrangement is pivotal because it minimises the cross-relaxation between Ln3+ ions27, which is a common issue in Ln-based or heavily Ln-doped phosphors. Optical investigations reveal theoretically and experimentally that 4f→5d transitions and Cl-to-Ln charge transfer transitions are the main light absorption mechanisms in Cs3LnCl6 (Fig. 1b, left panel). In contrast to the parity-forbidden 4f→4f transitions that typically limit the absorption abilities of Ln3+ ions, the 4f→5d transitions and Cl-to-Ln charge transfer transitions in these materials provide enhanced absorption in the near-ultraviolet region28. This theoretical insight explains the observed high photoluminescence (PL) performance, and lays the groundwork for tuning these transitions through careful compositional and structural modifications. For light emissions, the energy conversion mechanisms include 5d→4f and 4f→4f transitions according to the selection of Ln3+ ions (Fig. 1b, right panel). Among the various Ln elements explored (ranging from Ce to Lu, except Pm), Cs3TbCl6 has an extraordinary PL quantum yield exceeding 90.8%, significantly outperforming most lead-based perovskites and other Ln-based metal halides. Furthermore, under X-ray excitation, Cs3TbCl6 demonstrates an exceptionally high light yield of ~51,800 photons/MeV, which is more than double that of the benchmark commercial scintillator LuAG:Ce. These results affirm Cs3TbCl6 as a competitive scintillator for X-ray imaging. When integrated into a flexible thin film with polydimethylsiloxane, the Cs3TbCl6-based scintillators demonstrate promising X-ray imaging capabilities with a spatial resolution of 12 line pairs per millimetre (lp·mm−1). The high spatial resolution is important for applications requiring high-quality imaging, such as medical diagnostics, non-destructive testing, and security screening.
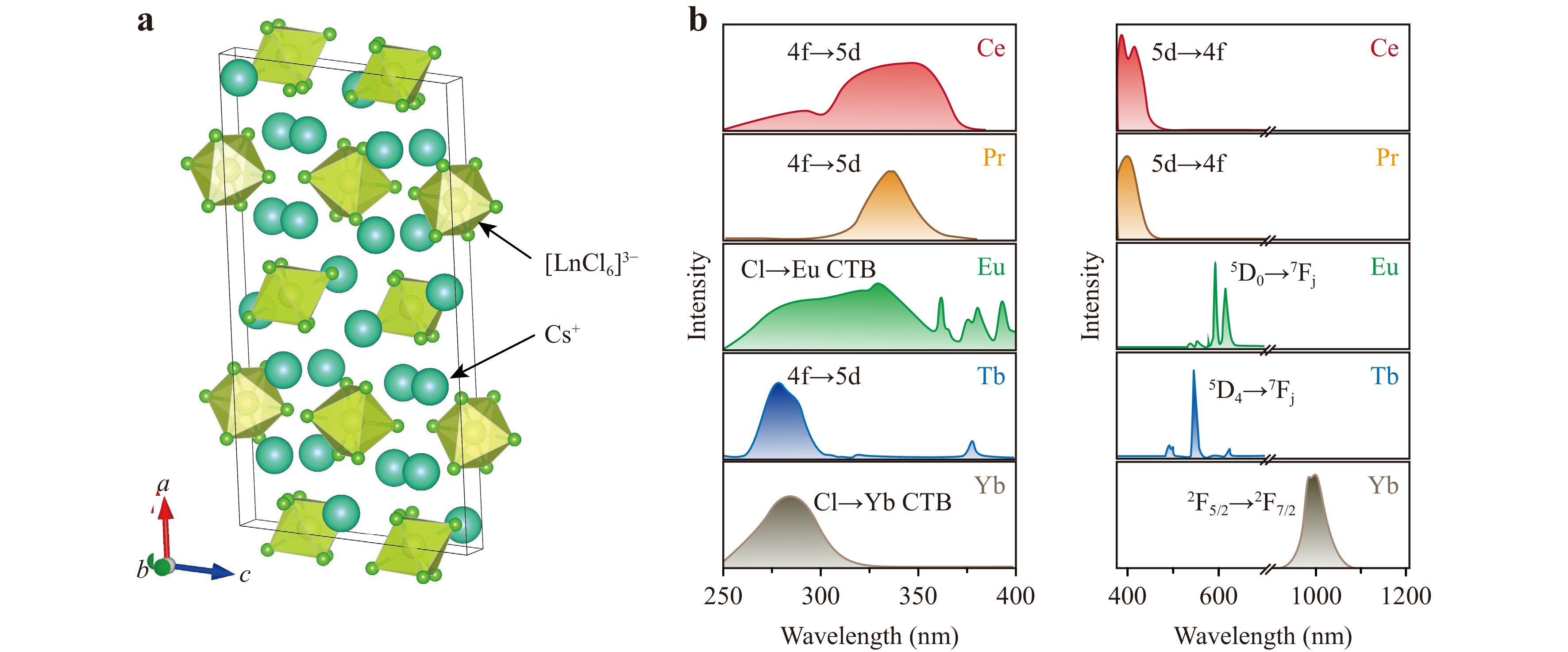
Fig. 1 Structure-function relationship of Cs3LnCl6 crystals. a Schematic of the unit cell structure of Cs3LnCl6 (Ln = Ce-Lu) crystals adopting a monoclinic C2/c space group. b The excitation (left panel) and emission (right panel) spectra of Cs3CeCl6, Cs3PrCl6, Cs3EuCl6, Cs3TbCl6, and Cs3YbCl6, respectively. The spectra were reproduced from Ref. 23.
This study opens up new directions for further studies. One of the challenges for metal halide scintillators, especially Ln-based scintillators, is their sensitivity to moisture29. Although the authors have emphasised the stability of Cs3LnCl6 under high temperatures or under constant irradiation, its moisture stability is questionable. Future efforts should be devoted to improving the encapsulation of scintillators, ensuring consistent performance in diverse environments. Another challenge for the Ln-based metal halide scintillators is their difficulty in integration with pixelated electronics. Moreover, the polycrystalline nature of Cs3LnCl6 microcrystals causes uncontrollable light scattering, thus unsuitable for light propagation management. To further improve the imaging quality, the development of directional growth for Ln-based metal halide scintillators with large single crystals that can be in situ seamlessly integrated with photodetector arrays will facilitate their adoption in practical X-ray imaging.
Ultrafast recrystallisation simplifies the synthesis of lanthanide-based metal halide scintillators
- Light: Advanced Manufacturing , Article number: (2025)
- Received: 28 March 2025
- Revised: 22 April 2025
- Accepted: 23 April 2025 Published online: 18 June 2025
doi: https://doi.org/10.37188/lam.2025.037
Abstract: An advancement in the fast synthesis of lanthanide-based metal halides at room temperature is proposed and demonstrated. The reported Cs3LnCl6 scintillators are potential candidates in X-ray imaging by providing a safer, more efficient, and environmentally benign alternative to address the long-standing concerns about toxicity and fabrication complexities of existing scintillators.
Research Summary
Ultrafast Recrystallization Simplifies the Synthesis of Lanthanide-based Metal Halide Scintillators
Scintillators transform incident X-ray photons into visible luminescence, a key step in modern radiation detectors for medical imaging and nondestructive industrial testing. Lanthanide-based metal halide scintillators, such as Cs3TbCl6, can produce intense, lanthanide-specific emissions under X-ray excitation. Hongjie Zhang and colleagues from Changchun Institute of Applied Chemistry now reported an advancement in the ultrafast synthesis of lanthanide-based metal halides at room temperature through a simple “recrystallization” approach, accomplished within one minute. Under X-ray excitation, Cs3TbCl6 delivers an exceptionally high light yield of ~51,800 photons/MeV, more than twice that of the commercial benchmark LuAG:Ce scintillator. As a result, high-quality X-ray imaging with a spatial resolution of 12 lp·mm⁻¹ can be achieved.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article′s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article′s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.






 DownLoad:
DownLoad: