-
The diversity of biological threats and their potential risks to human health have prioritised the development of precise and efficient elimination technologies in the biomedical field1. Conventional approaches, including antibiotic therapy, surgical resection, and immunotherapy, while showing some efficacy, present several limitations. For instance, the overuse of antibiotics leads to heightened drug resistance, surgical interventions carry invasive risks, and immunotherapy can trigger excessive inflammatory responses due to off-target effects2. Moreover, emerging issues such as nanoplastic pollution, the spread of drug-resistant bacteria, and cancer cell metastasis have escalated in recent years, creating an urgent need for innovative technologies that offer precision, low toxicity, and active navigation capabilities3.
Rapid progress in biomedical engineering and nanotechnology has positioned microrobots at the forefront of precision medicine owing to their small size, high manoeuvrability, and minimal invasiveness4. These microrobots can perform complex tasks at the microscopic level, including targeted drug delivery, tissue repair, and pathogen eradication. However, traditional microrobots largely rely on magnetic or chemical propulsion systems, frequently necessitating modifications with external materials (e.g. magnetic nanoparticles), which pose challenges such as reduced biocompatibility, immune rejection, and limited ability to penetrate biological barriers5. Moreover, existing technologies lack precise control over robotic functionalities, limiting their application in complex physiological environments6.
The emergence of optical manipulation technologies offers transformative potential in this field. Light provides non-contact operation, high spatiotemporal resolution, and programmability, enabling precise regulation of cellular functions and real-time navigation of microrobots7. By utilising near-infrared (NIR) light or laser beams, researchers can remotely activate cellular activities, guide movement trajectories, and modulate cellular metabolism to perform tasks within intricate biological settings8. Recently, light-controlled microrobots have become a prominent research focus, particularly “biological microrobots” that employ natural immune cells, such as macrophages, as carriers9. These systems attract significant attention due to their inherent compatibility with the human immune system.
Li et al. developed a light-controlled phagocytic macrophage microrobot (phagobot) that activates resting macrophages through localised near-infrared (NIR, 1064 nm) micro-irradiation (Fig. 1a), eliminating the need for genetic modification or external materials while enabling controllable phagocytic capabilities10. This microrobot achieves precise translational and rotational motion by optically manipulating macrophage filopodia, attaining speeds of 3.4 µm/min in vitro and 4.3 µm/min in vivo, and demonstrating the ability to track complex trajectories (Fig. 1b). Experiments confirmed that in vitro, phagobots can target and engulf bio-threats (e.g. yeast cells, micro/nanoplastics, S. aureus bacterial cells), with phagocytic efficiency dependent on target size and laser power (Fig. 1c). In vivo, phagobots successfully infiltrated the intestinal tissues of zebrafish larvae to eliminate cellular debris, showcasing their excellent biocompatibility as shown in Fig. 1d. This technology combines precise optical manipulation with the innate immune functions of macrophages, offering innovative strategies for precision immunotherapy, infection control, and environmental pollutant removal while avoiding the biocompatibility challenges linked to conventional magnetic propulsion or genetic engineering approaches.
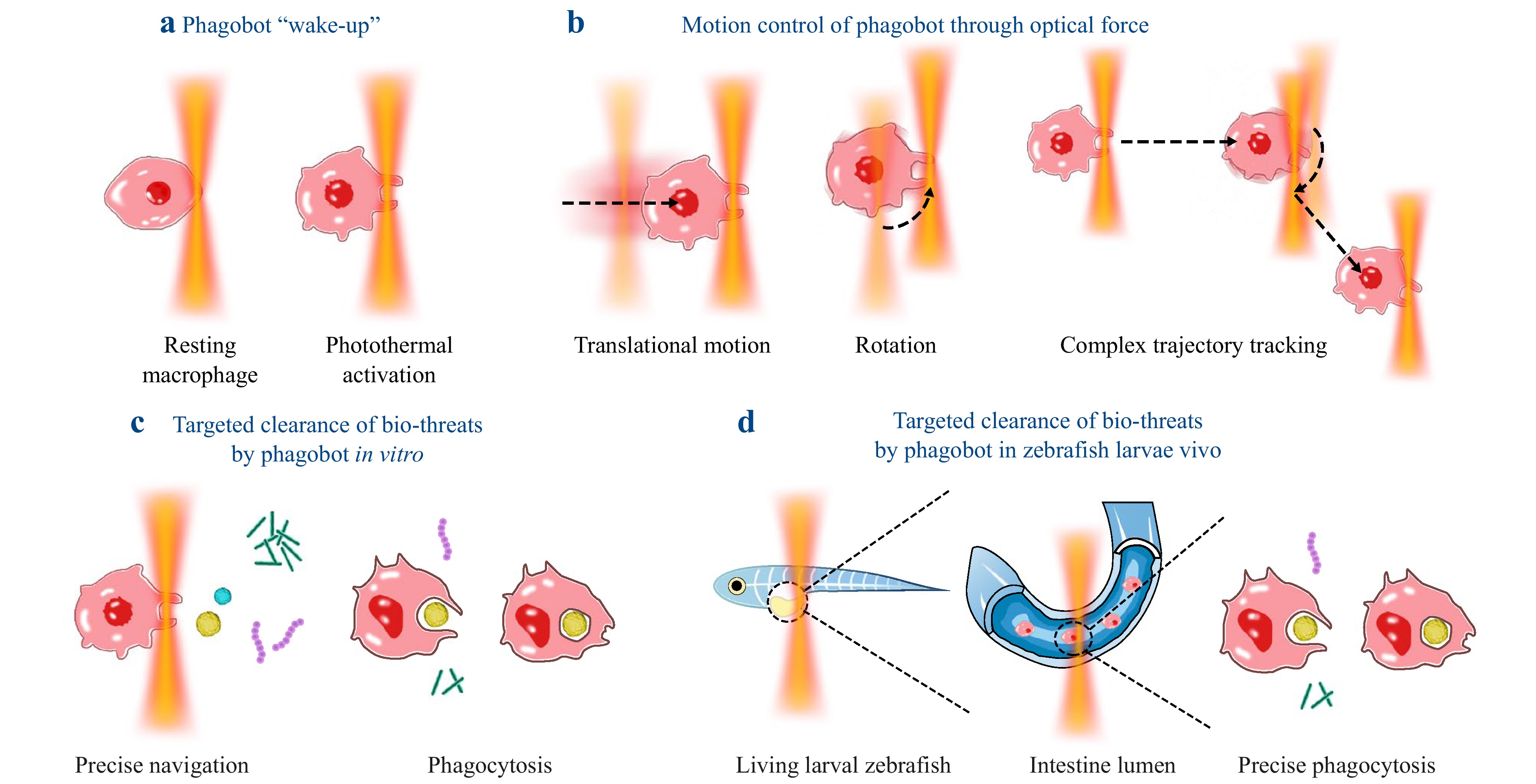
Fig. 1 Light-controlled phagocytic macrophage microrobot (phagobot) for intelligent bio-threat elimination. a NIR optothermal activation of phagobots in resting macrophages. b Optical manipulation guiding activated phagobots along predefined trajectories. c In vitro targeted bio-threat clearance by phagobots. d In vivo targeted bio-threat removal in zebrafish larvae.
Light-controlled biological microrobots embody a significant convergence of biomedicine and nanotechnology, utilising the natural functions of immune cells alongside precise optical manipulation to intelligently eliminate biological threats3. Current studies have confirmed their distinct benefits in targeted delivery, lesion eradication, and microenvironment modulation through in vitro experiments and small animal models. However, advancing the technology requires interdisciplinary collaboration to tackle key challenges, including deep-tissue manipulation, diversification of immune cell carriers, and large-scale production. Future incorporation of ultrasound positioning11, optofluidic effects12, and flexible fibre-optic implantation13,14, may allow these microrobots to surmount optical penetration depth limitations, greatly improving their 3D navigation accuracy and penetration efficiency in deep tissues, thus advancing their use in tissue navigation and clearance. Moreover, extending the platform beyond macrophages to include various immune carriers (e.g. neutrophils15) or biocompatible/biodegradable carriers16, along with dynamic trajectory planning, holds promise for targeting multiple lesions, eradicating drug-resistant pathogens, and environmental remediation.
Light-controlled biological microrobots precisely eliminate bio-threats
- Light: Advanced Manufacturing , Article number: (2025)
- Received: 19 May 2025
- Revised: 19 August 2025
- Accepted: 20 August 2025 Published online: 24 October 2025
doi: https://doi.org/10.37188/lam.2025.069
Abstract: Light-controlled biological microrobots employ near-infrared-activated macrophages to achieve precise navigation and targeted elimination of biological threats, such as micro/nanoplastics, yeast cells, S. aureus bacteria, and cellular debris, without the need for genetic modification. These biological microrobots enhance approaches to immunotherapy, infection control, and environmental remediation, all while overcoming conventional biocompatibility constraints.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article′s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article′s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.






 DownLoad:
DownLoad: