-
Tissue engineering (TE), as a modern scientific discipline, was first defined in 1993 by Langer as “the principles of biology and engineering to the development of functional substitutes for damaged tissue”1. Over the past 30 years, the development of TE has greatly benefitted from the leap of progress in areas such as biomaterials, three-dimensional (3D) printing technologies, integration of nanotechnology, stem cell technologies, and gene-editing technology. As such, the application of TE has obtained remarkable results in repairing bone defects, skin burns, nervous system, cornea replacement, cartilage, vascular disease, cosmetic procedures etc. It is widely believed that clinical medicine will be significantly transformed by the power of TE in the foreseeable future2, 3. In Fig.1, the triad concept defines TE as: (1) the cells to produce the desired tissue, (2) the scaffolds to provide a framework and initial support for tissue growth, and (3) the signals in the form of biochemical or environmental (physical or chemical) cues, i.e., cell growth factors, that affect the cell’s growth and phenotype4-6. Generally, TE starts with the seeding of variable cells and biological signals into a 3D scaffold, followed by adhesion, proliferation, and differentiation of cells incapsulated in the scaffold. Afterwards, the scaffold is implanted in vivo to promote tissue regeneration7.
TE is a process of generating tissue by adding the combination of appropriate cells and materials onto the specific scaffolds7. However, most normal tissue-derived cells, except for certain cells such as blood cells, are anchorage-dependent and reside in the extracellular matrix (ECM)8, 9. Further details on ECM can be found in Refs. 9, 10. Ideally, the best 3D scaffold for a given engineered tissue should be the corresponding ECM in its native states. Nevertheless, native ECMS are functionally diversified with complex composition, and naturally dynamic, making it extremely challenging to mimic such structures in vitro9. Therefore, a thorough investigation into scaffold fabrication is critical to enhance TE development9, 11, particularly in the fabrication of scaffolds that mimic, if not completely but at least partially, the functions of native ECM, without compromising the cell and tissue compatibility, bioactivity, and mechanical properties12, 13.
An ideal 3D scaffold that assists cell migration and in-filtration requires a highly porous architecture, well-interconnected pore networks, and consistent and adequate pore size3, 12. Over the past few decades, 3D scaffolds have been fabricated via various conventional manufacturing techniques, e.g., fiber bonding11, phase separation14, solvent casting15, and membrane lamination16. However, these techniques all fall short of a precise control over the scaffold architecture, pore network, and pore size. Thanks to the leaping progress in 3D printing technology in the past 20 years, the fabrication of customized scaffolds with controlled structure and scale is now a reality17-23. Moreover, advanced 3D printing methods have enabled the printing of the fixture of cells, growth factors, and multiple biocompatible materials (both natural and synthetic) altogether onto the complex 3D scaffolds that possess a high degree of structural and functional similarities to native ECMs2. Fig. 2 presents some examples of 3D scaffold fabrication via different methods.
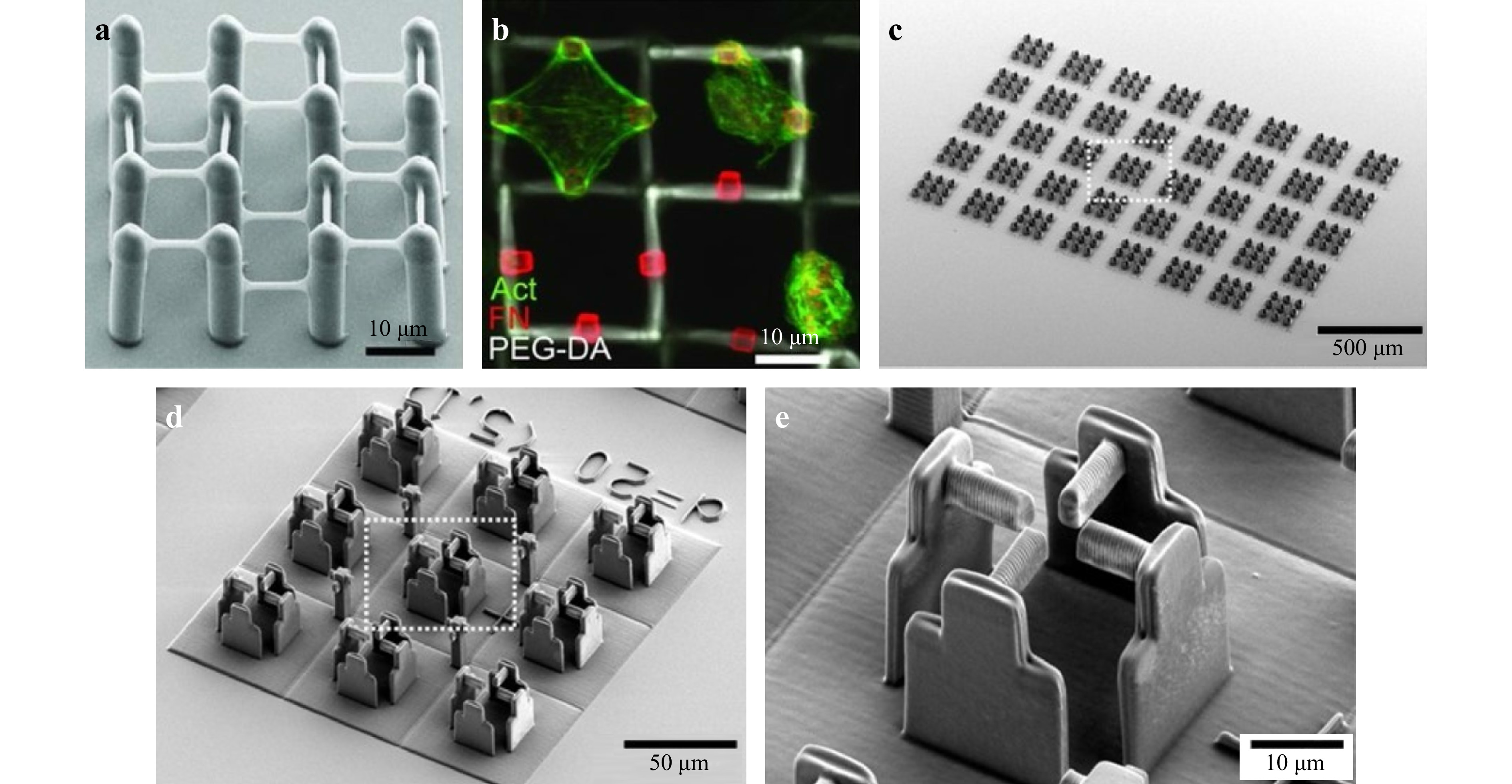
Fig. 2 a-b Cell holder (reprinted by permission from Wiley-VCH: Advanced Materials24, copyright 2011). c-e Scanning electron microscopy (SEM) micrographs of a typical sample with 360 scaffolds (without the host-guest hydrogel) with increasing magnifications from left c to right e. (reprinted from AAAS: Science Advances25, copyright 2020).
There are two distinct types of 3D printing methods: non-optical and optical26. The non-optical-based methods include techniques such as fused filament deposition (FFD), fused deposition modeling (FDM), electron beam melting, powder bed fusion (PBF), and partial extrusion-based printing (such as binder jetting). On the other hand, the optical-based methods include stereolithography (SLA), selective laser sintering (SLS), multiphoton stereolithography, and other extrusion-based and jetting-based printers. More details on these 3D printing methods can be found in earlier publications27, 28.
The optical method overpowers its counterpart for its fabrication resolution, quality, reproducibility and rate as summarized below29:
1. As the fabrication resolution via the optical approach is determined mainly by the diffraction limit of the optical system, one can obtain a higher resolution by using a light source with shorter wavelengths and an objective with higher NA.
2. As opposed to other methods based on heat treatment and laminating, the optical method renders a firmer connection of adjacent voxels, and its post-processing step, such as photocuring, also contributes to the printing quality.
3. The optical 3D printing method is more effective in fabricating various materials, e.g., metals and alloys, ceramics, polymers, and composites, which is an important strength in scaffold fabrication where a material’s specific nature, physical and chemical properties are called for to address different needs.
Based on the above advantages, the optical 3D printing method is undoubtedly the preferred method for scaffold fabrication in TE18.
In the following sections, we first provide a comprehensive review of various optical 3D printing methods for fabricating 3D tissue scaffolds from the perspectives of the fundamentals (Section 2), materials (Section 3), and applications (Section 4.1). Secondly, we compare the performance across different fabrication technologies in terms of precision, rate, materials, and application scenarios. Finally, we recommend the most situation-appropriate method under different application scenarios (Section 4.2), followed by an outlook for the research focus in the coming years (Section 5).
-
Optical 3D printing methods are implemented by connecting voxels and building 3D structures via light-matter interactions, which involve different physical processes, such as melting and fusing materials by heating, curing, or sintering; photo-induced cross-linking; and transferring materials via laser assisted bio-printing, etc.30, 31.
Common techniques are as follows:
1. Optical extrusion-based methods, whereby the photosensitive materials are extruded and then solidified by light exposure (such as UV). A good example is direct ink writing (DIW)32, 33.
2. Optical jetting-based methods, whereby droplets of feedstock materials are selectively sprayed and deposited, followed by light curing. Common methods include material jetting (MJ)34, 35 and polyjet printing (PJP)36-38.
3. Laser-based methods, whereby laser is used to bond material powders, solidify fluid media39-42, or induce cross-linking mechansms. Common techniques include SLA43-45, SLS46-48, and two-photon polymerization (TPP)49, 50.
In recent years, the 3D printing technology has advanced significantly thanks to the innovations brought about by the latest optical methods. In the next section, we will elaborate on these 3D printing methods, from their fundamental principles to the most advanced development.
-
Extrusion-based 3D printing methods play a critical role in the additive manufacturing (AM) technology due to their strengths in reproducibility, flexibility, and process control30. Most extrusion 3D printers are thermoplastic-based, where heated thermoplastic material, in the form of paste or thick ink, is extruded from a moving nozzle head to print 3D objects layer by layer. In recent years, extrusion 3D printing systems in TE have gained much attention due to its simple design and its capability to process a wide range of biomaterials effectively. In certain applications, such as tissue scaffold printing, additional depositing and fusing strategies need to be applied as per the materials being used. Most common extrusion-based fabrication methods include FDM, precision extrusion deposition (PED), DIW, PJP, binder jetting etc. Here we will review only light-based techniques such as DIW and PJP. Details on other extrusion-based 3D printing methods can be found in Refs. 30, 51.
DIW is one of the most prevalent extrusion methods in printing at meso- and micro-scales. Conventional DIW methods involve printing with melted materials ("ink"), i.e., polymer and wax, that is dispensed from small nozzles under controlled flow rate and then deposited along digitally defined paths to fabricate 3D structures in a line-by-line or layer-by-layer manner32. Recent research has focused on addressing the growing need for material diversity and biocompatibility. In 2010, Lebel et al. developed a UV-assisted DIW fabrication system that enabled continuous fabrication of 3D geometry52. In this system, the UV-curable nanocomposite is exposed to 365-nm UV light immediately after extrusion to enable free-form printing. However, the resolution obtained is limited at around 100 μm. Based on the same setup, a wide spectrum of materials have been printed53-56. In 2016, Mark et al. proposed a laser-assisted DIW, namely laser-DIW, to achieve one-step fabrication of complex 3D metal features on different substrates33. As the schematic shown in Fig. 3a, the UV light was replaced by a focused infrared (IR) laser with sufficient power density for metal annealing. By combining the patterning and annealing in a single step, the metallic features were printed with the requisite mechanical properties to fabricate arbitrary objects in midair with precision, hence generating complex curvilinear structures without the need for support material. The resulting metal wires (with diameters of 10 μm, 3 μm, and 600 nm) could be deposited on low-cost and soft plastic substrates such as polyethylene terephthalate (PET) films. Such method has opened up tremendous possibilities in fabricating biomedical sensors or other customized electronics in an innovative fashion.
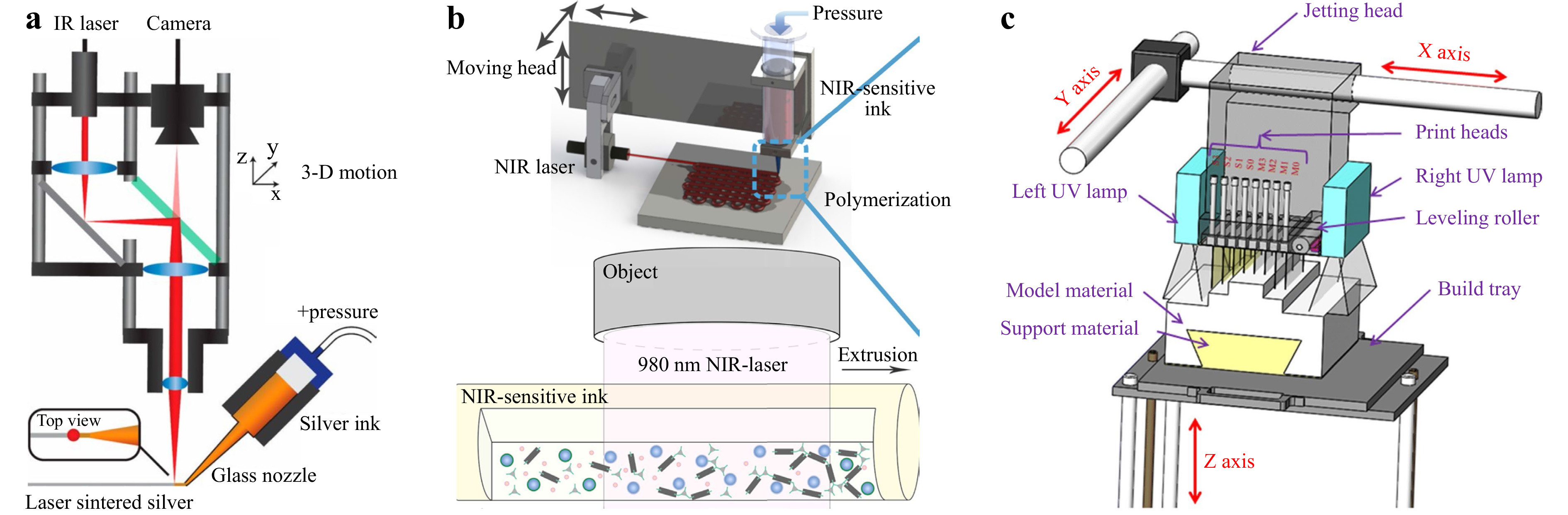
Fig. 3 Light-assisted extrusion 3D printing schemes. a Laser-assisted printing of 3D metal structures. (reprinted from National Academy of Sciences: Proceedings of the National Academy of Sciences33, copyright 2016). b UCNP and NIR laser enabling a curing length of over 10 cm (reprinted from Springer Nature: Nature Communications57, copyright 2020). c Typical PolyJet printing setup (reprinted from Springer Nature: The International Journal of Advanced Manufacturing Technology61, copyright 2019).
Traditional UV-based DIW techniques controls the printed feature size by varying the power of the UV light; yet, as the curing light power increases, the resolution and structural uniformity care be significantly compromised, making multiscale parallel processing challenging. To overcome such difficulty, in 2020, Zhu et al. combined a near-infrared (NIR) laser with up-conversion nanoparticles (UCNP), as shown in Fig. 3b, and realized a curing length of over 10 cm57. Owing to the considerably improved NIR laser penetration depth, this NIR-DIW method did not only solidify the deposited filament with a diameter up to 4 mm, but also demonstrated parallel manufacturing capability, which could be integrated into existing DIW systems to streamline the overall printing process.
-
Optical jetting-based methods are an accurate AM technique in which photocurable droplets of build materials are selectively sprayed and deposited to form a designed 3D structure58, 59. One of the most commonly used material jetting methods is PJP. As opposed to typical DIW printers, a PJP printer equipped with multiple nozzles, as shown in Fig. 3c, fabricates parts by jetting thousands of photopolymer droplets onto the build platform and solidifying them with UV light. These nozzle heads repeat the extrusion process, layer by layer, until the 3D printing process is complete60, 61. PJP can build models with relatively high resolution and fast molding speed62-65, and its multi-material printing capability has allowed sacrificial supporting structures in the finished model and improved the topological design for the printed device63. Nevertheless, the sandwich layout of the nozzle and curing lamp makes it difficult for advanced optical devices to be integrated. Therefore, recent research has more focused on enhancing the material and design. For instance, Liu et al. introduced a snap-fit method into polymer lattice design that allowed for a large-scale support-less lattice fabrication58. Childs et al. obtained a similar support-less result with a design where the target model was divided into two halves with an easy integration feature65. In contrast to the traditional approach, such design not only reduced the post-processing time by approximately 98% but also expands the possibility for broader PJP applications in 3D micro-printing.
-
SLA is regarded as the first 3D printing technology in the 1980s66. Today, SLA, with an industry share of approximately 11% in 2021, prevails all other conventional printing technologies. SLA fabricates 3D objects based on layer-upon-layer deposition and polymerizes photosensitive resin via laser beams. SLA can be categorized into scanning (Fig. 4a) and projection (Fig. 4b) systems, based on how the laser is delivered.
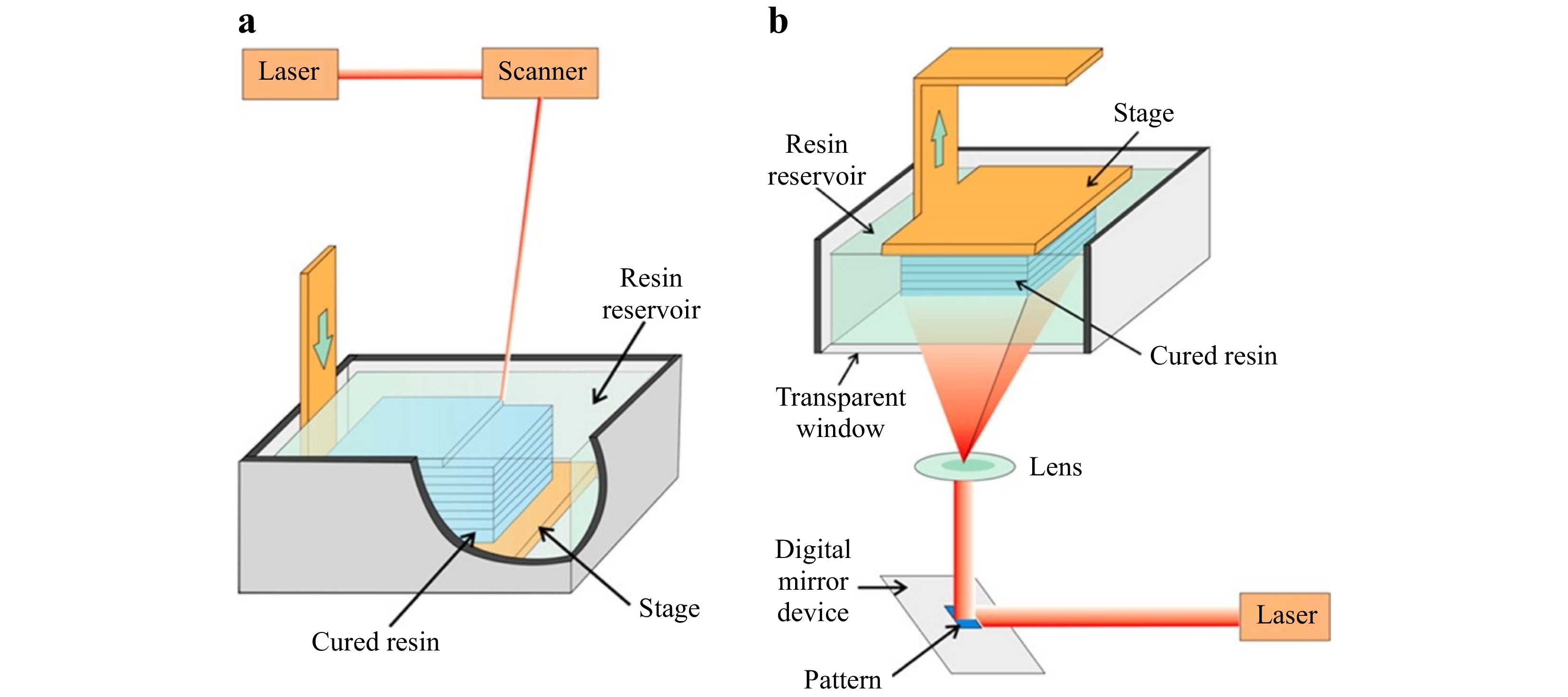
Fig. 4 Schematic illustration of SLA: a Scanning stereolithography, and b Projection stereolithography (reprinted from ACS: Analytical Chemistry67, copyright 2014).
In the scanning system68, 69, the stage is located below the liquid resin surface. A single laser sweeps along the resin surface, point by point and line by line, until the desired layer is completely cured. One needs to bear in mind that the thickness of the cured resin is subject to many factors, such as the exposure time, scanning rate, and laser power. To initiate the following layer, the stage sinks further into a vat until a new layer of liquid resin covers the surface and a new curing process begins69, 70. The above process repeats itself until the whole 3D object is completely printed. However, the size of the vat constrains the height of the desired object; yet a large vat can cause additional resin waste and cleaning procedures, and besides, the serial scanning process is time consuming. For small or meso-scale printing, an inverted optical design is often adopted to achieve a more compact system envelope, which eliminates the height constraint of the printed structures and uses resins more efficiently71. Owing to the inverted nature, more support structures are required for certain 3D objects, which may incur additional post processing time.
The projection system, also classified as digital light procession (DLP) in some literature68, 72, 73, consists of the same components as the scanning system, but with a different mechanism. The digital micromirror device (DMD) in the projection system allows the whole layer to be cured simultaneously, i.e., layer-by-layer74, 75. With DLP, the movable stage is suspended above the resin reservoir, and the light source is located under the vat. Such configuration not only removes the height restriction on the printed parts but also demands a lower volume of resin67, 76. Though the DMD-based projection system applies the same fundamentals as the scanning system, it greatly reduces the production time due to the simultaneous use of millions of mirrors in a DMD, which cures the entire layer with a single exposure.
Among all the advanced SLA techniques, the projection micro stereolithography (PμSL) is considered a 3D printing technology that fabricates complex 3D architectures with multiple scales and materials at high resolution (up to 600 nm67, 77)77-79. Fig. 5a shows a large area projection micro-stereolithography (LAPμSL) developed by Zheng et al. In their design, a traditional DMD-based PμSL system was integrated with a coordinated optical scanning system. By combining a galvanometric mirror with a customized scanning lens to project the light pattern from the DMD onto the UV curable polymer resin surface, microstructures were fabricated within just a few hours of time, with feature sizes spanning over four orders of magnitude78. In 2018, Yang et al developed another PμSL, i.e., immersed surface accumulation-based 3D (ISA3D)80. By combining a dynamically controlled light beam projection with a five-axis light-guiding tool, the ISA3D printing system fabricated microstructure with 2.5 μm feature size on curved surfaces at centimeter scale. In 2020, BMF Materials Technology Co, Ltd., commercialized the PμSL system as in Fig. 5b. By adopting the multiple projection stitching process, the system offered two printing options with different resolutions and sizes: (1) 2 μm per pixel with a 50 mm × 50 mm printing area, and (2) 10 μm per pixel with 94 mm × 52 mm printing area80. It is worth noting that Aftab et al. proposed a high-resolution SLA with multi-scale microstructures with feature sizes ranging from 16 μm to thousands of micrometers by utilizing a static inert immiscible liquid below the resin as the constraining interface81. Also worth mentioning is a rapid SLA, proposed by Wakler et al., which delivered continuous printing over large areas at approximately 100 liters per hour82. This is the 3D printer of the largest scale ever reported in all publications. By combining liquid-crystal displays (LCD) and light-emitting diode (LED) sources, a low-cost (< US$ 500) 3D printing system has been developed, where the printing resolution is determined by the LCD pixels (20 - 50 μm); the fabrication rate is relatively low due to the low transmissivity of LCD screens77, 83.
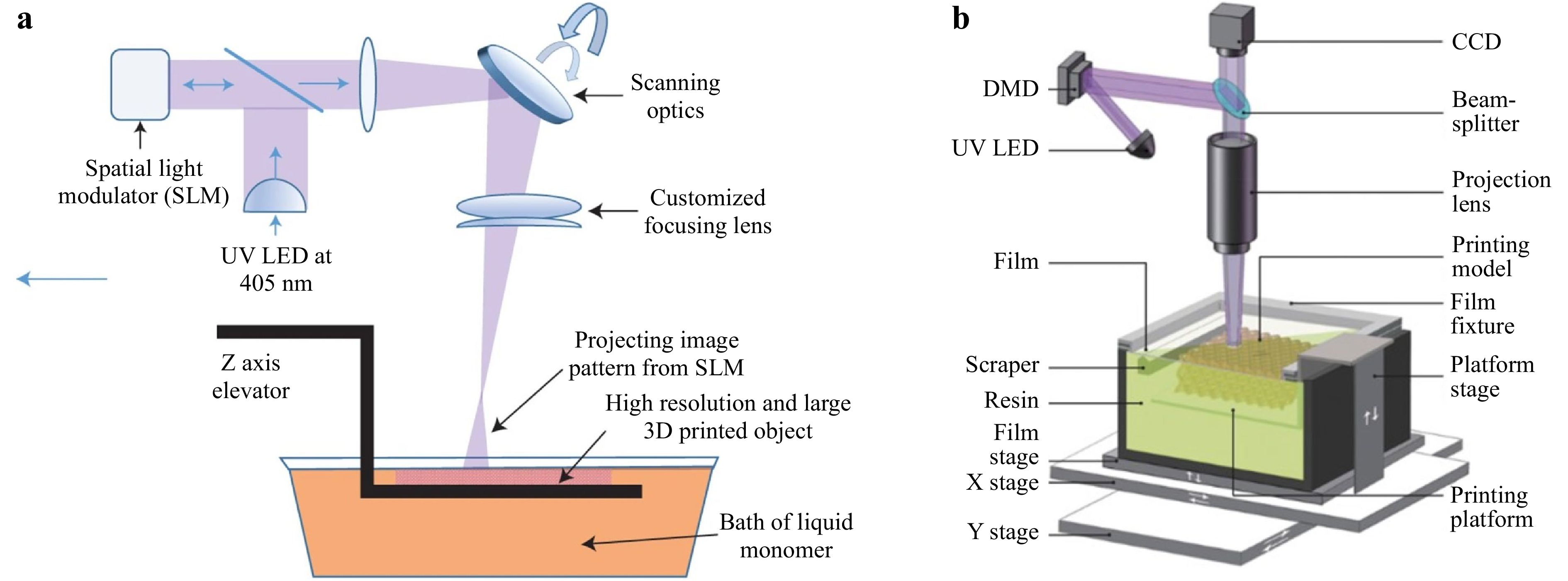
Fig. 5 a Schematic of the LAPμSL printing system that combines a traditional DMD-based PμSL system with a coordinated optical scanning system (reprinted from Macmillan Publishers: Nature materials78, copyright 2016). b Schematics of LAPμSL system (reprinted from Elsevier: International Journal of Extreme Manufacturing79, copyright 2020).
In general, SLA-fabricated parts feature relatively high dimensional accuracy, intricate details, and smoother surface finish, making them ideal visual prototypes. However, the SLA printing process is more complicated since it necessitates support structures which need to be removed by post-processing. Besides, SLA materials are more brittle than other 3D printing materials, which is a drawback to be solved by future development in advanced materials.
-
SLS is an AM technique that uses high-power laser to sinter small powder particles into solid 3D structures, which for example can be used to print bone scaffolds. SLS was first introduced in the early 1980s84 and developed by Carl Deckard and Joseph Beaman in the mid-1980s85. Its schematic is shown in Fig. 6.
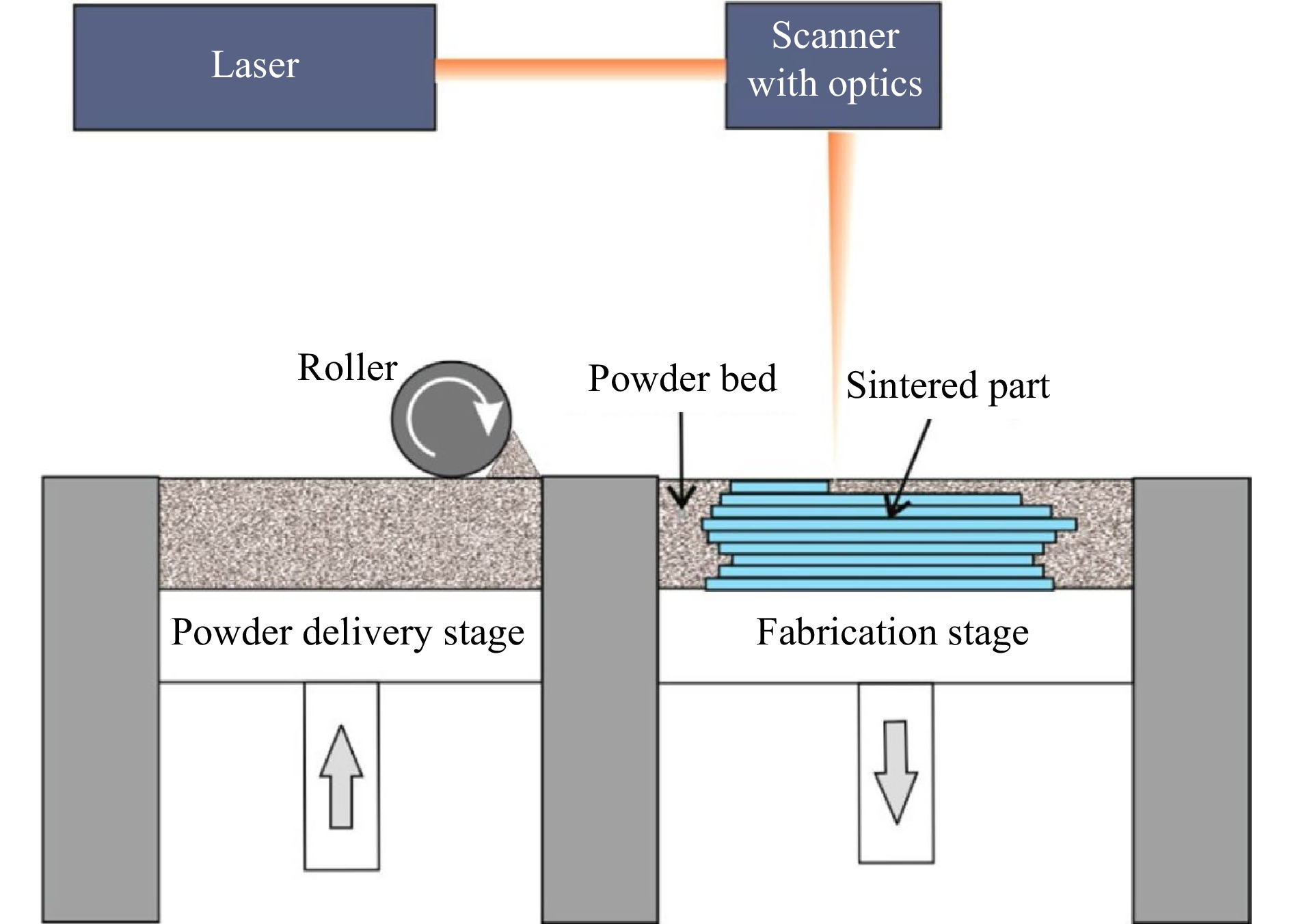
Fig. 6 Schematic of an SLS 3D printer (reprinted from ACS: Analytical Chemistry67, copyright 2014).
SLS shares many similarities with SLA, but rather than using resin in liquid form, SLS uses thermoplastic polymers that come in granular or powder form. Conventional SLS process includes three steps:
1. Printing: The powder is dispersed in a thin layer on top of a platform in the build chamber. By raising the temperature, the laser selectively sinters the powder surface to the melting point, and then exploits diffusion to build the parts layer-by-layer. The printing platform subsequently moves downward and fabricates a new powder layer. The process repeats itself until the whole part is complete.
2. Cooling: After printing, the build chamber cools down slightly, first inside the print enclosure and then outside the printer, so as to ensure optimal mechanical properties and to prevent warping.
3. Post-processing: The finished parts are taken out from the build chamber, separated, and removed of excess powder with compressed air or other blasting media. Unsintered powder is recycled.
SLS parts are ideal functional parts and prototypes owing to their isotropic mechanical properties. Additional support structures are unnecessary since the unsintered powder supports the part during the printing process and as a result, designs with complex geometries and shapes can be easily fabricated. Moreover, SLS enjoys a wide range of processable materials, from polymers such as polycarbonate (PC), polyvinyl chloride (PVC), acrylonitrile butadiene styrene (ABS), nylon, resin, and polyester to metal and ceramic powders. With regard to the resolution, several parameters need to be considered, e.g., the laser power and the size of the powder material. The common layer thickness is 50-200 μm, subject to micro-scale powder (typically 5-20 μm) and the laser spot size86, 87. However, SLS parts suffer a certain degree of grainy surface finish and internal porosity, so post-processing may be necessary if surface smoothness or water tightness is required.
To overcome these limitations, many advanced methods are thus developed88, 89. For example, Nilabh et al. proposed a novel microscale SLS process technique, namely μ-SLS, which produced 3D metal objects with sub-5μm resolution and a throughput of greater than 60 mm3/h90. One of the modifications included the use of nanoparticle (NP) inks in lieu of the microscale powder for conventional SLS process. Other improvements included an optical sub-system, where laser light was patterned via a digital micromirror array, with each pixel scaled down to nearly 1 μm.
To sum up, SLS techniques can be widely applied due to its design freedom, high productivity and throughput, low cost per part, and proven end-use materials in the market91. Further study on the comparison of the current commercial SLS 3D printers can be found in Ref. 92. In addition to the abovementioned laser-based 3D methods, other widely applied methods in microscale AM are all based on laser-material interaction, including Laser Chemical Vapor Deposition (LCVD93), laser-enabled electrochemical printing (LECP), laser-induced forward transfer (LIFT), and holographic optical tweezing process (HOTP)94-98. Further details on these laser-based 3D printing methods can be found in other publications88.
-
TPP, another sought-after 3D printing method, resorts to ultrashort laser pulses and nonlinear optical absorption process99. In the liquid photoresist, the photoinitiator absorbs two photons simultaneously to release a free radical that initiates the chain reaction for polymerization within the focal volume of the ultrafast laser, enabling 3D resolved nanofabrication100. Following the laser irradiance and subsequent development, i.e., washing out the non-illuminated regions, the polymerized material remaining in prescribed 3D forms reaches a resolution of 100 nm or above101, 102. In accordance with the dimension of the printing element for single exposure, different TPP systems can be created to connect voxels and print 3D structures by point-scanning, multi-point-scanning, or layer-scanning.
Though a point-scanning TPP-based 3D printing system is easy to set up with high resolution, the practicality is compromised by the low fabrication rate. An early example is a bull sculpture with an accuracy of 120 nm printed via raster scanning103. Point scanning can be realized via other methods, e.g., moving the sample stage104; based on this approach the first 3D micromechanical component, i.e., a helix of 1.3 μm in width, was fabricated. In this design, the fabrication speed is determined by the stage. To increase the scanning speed, a common solution is to use galvanometers to perform the x- and y-axis scanning and then move the stage, e.g., the piezo stage, to change the height of the z-axis105, 106. As such, the inertia is lower than that of the mechanical stage, thus increasing scanning speed and the control. In addition, polygonal mirrors or resonant scanners can also improve the scanning speed. For example, in 2019, Pearre et al. proposed a novel scanning method where a commercial resonance mirror was operated at 8 kHz107 to achieve a scanning speed of 8 × 103 mm/s. The smallest feature size reached the order of microns. Nevertheless, the application of such design was limited owing to certain technical barriers; for example, the synchronization between a scanning device and light-switch device (e.g., electro-optic modulator) for fabricating complex 3D structures.
The fabrication speed becomes particularly important when fabricating large-volume objects. Here multi-point parallel scanning proves to be an effective method to increase the throughput. For example, one can use a micro-lens array to generate multiple laser focal points for parallel processing, while a stepping motor and a linear actuator are used for 3D scanning108. However, the challenge is to calibrate multiple focal points at the same time, and besides, the fixed laser focus array cannot fabricate aperiodic structures.
An alternative method to divide the incident beam into several beams is via the use of static or programmable optical diffractive elements109, 110, e.g., liquid crystal-based spatial light modulator (LC-SLM). As such, multi-point printing can be achieved by moving the stage and modulating the LC-SLM synchronously. For example, Maibohm et al. used an SLM to generate nine laser beams for parallel processing111. A large periodic and high aspect ratio array structure was fabricated at a writing speed of 15 μm/s and a fabrication area of several hundreds of μm2. The finished structure was used for cell culture experiments. However, the limited LC-SLM switching rate, i.e., tens of Hertz, had an adverse effect on the printing speed and the fabrication of complex or aperiodic structures.
To achieve speed and flexibility simultaneously, or even random scanning, the DMD overpowers the SLM owing to its high pattern rate, i.e., up to 32.5 kHz, and power handling ability. When it comes to femtosecond laser imaging, the DMD-based single-point scanning and beam shaping capabilities have been proven to be highly effective112. Moreover, commercial DMDs are well-designed and cost-effective. Lastly, DMD-based fabrication techniques can perform random-access scanning at any position within the operational range, not to mention its high precision and high throughput112-114. After thoroughly investigating into the topic, our group proposed a novel multi-focus scanning method using DMDs in 3D nano-manufacturing in 2019115. This DMD-based scanning method can randomly generate from 1 to 100 scanning points in space, with axial and lateral scanning resolutions of 270 nm and 130 nm, respectively. Fig. 7a shows a schematic diagram of the light path of this multi-focus 3D printing system. Fig. 7b shows the printing result achieved by single-point scanning, the design of the octet truss model, and the obtained SEM image. Fig. 7c shows the scanning results of three woodpiles structures manufactured with a single focus, two foci, and three foci, respectively. The maximum parallel printing speed is 22.7 kHz or 5 mm/s. When a 40 × objective lens is used, the working volume is 103 × 206 × 524 μm3.
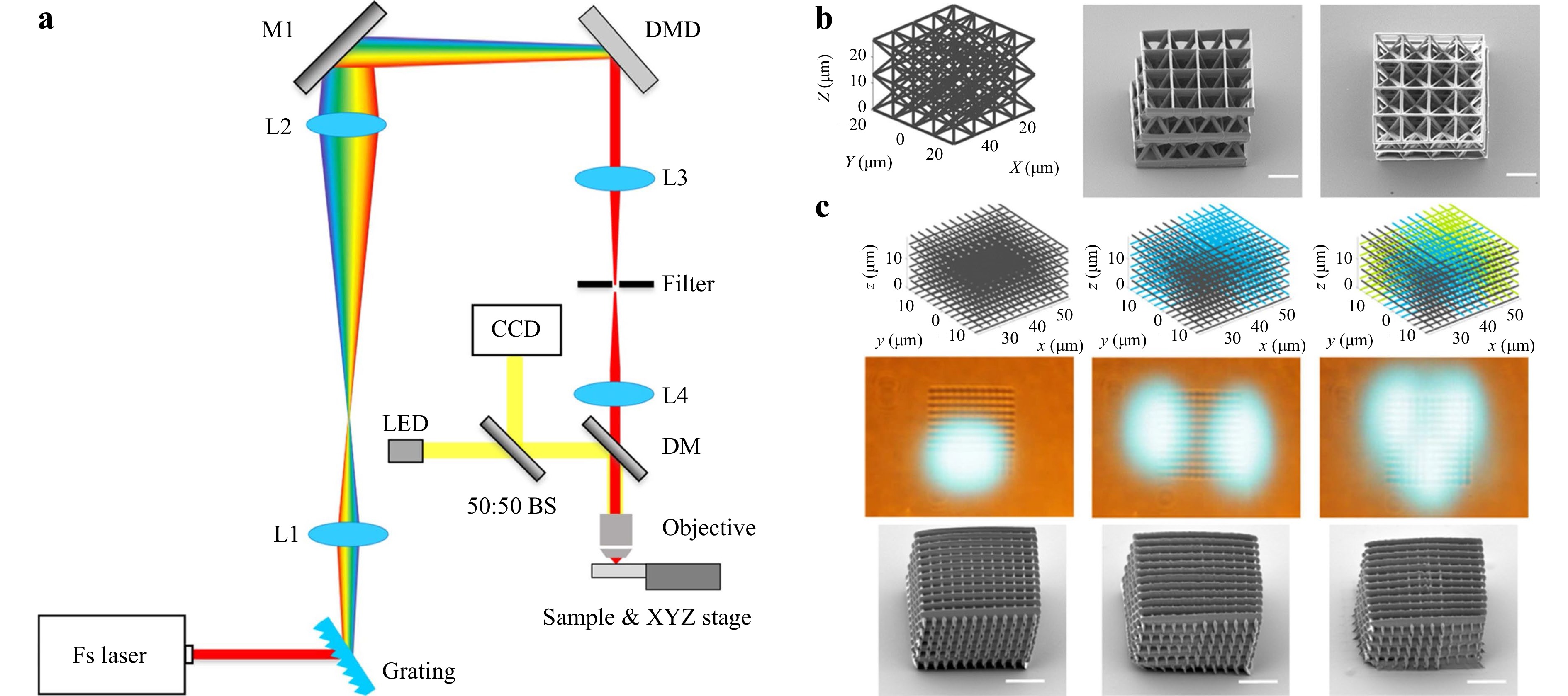
Fig. 7 a Configuration of DMD-based multi-focus scanning system. b DMD-based single-point scanning printing design model and SEM image of the octet truss printing result. c DMD-based scanning with a single focus, two foci, and three foci respectively to make woodpile structures (reprinted from Springer Nature: Nature Communications115, copyright 2020).
Compared with single-point and multi-point scanning, the layer-by-layer scanning method reduces fabrication time at the expense of increased minimum feature size77, 116. Although the early layer-scanning optical method, such as the PμSL in Section 2.2.1, features rapid processing time77-79, the fabrication performance is greatly affected, i.e., disrupted speed caused by the back-and-forth switching and compromised resolution as a result of limited number of pixels. Here TPP comes into play to with its capabilities to improve processing accuracy and generate nanoscale features.
At the same time, simultaneous spatial and temporal focusing (SSTF) is proposed to disperse the laser pulses in space through a diffraction grating, and then recombine the dispersed spectrum, i.e., compress the laser pulses, at the conjugate plane, which effectively forms a femtosecond light sheet at the focal region117, 118. The advantage of this method is that SSTF enables TPP to be performed in a layer-by-layer fashion without sacrificing the resolution. Temporal focusing has been demonstrated for both high-speed imaging and fabrication with slightly compromised resolution117-120. In 2019, our group proposed a femtosecond projection two-photon lithography (FP-TPL) method where a DMD was combined with temporal focusing to encode arbitrary 2D patterns on the light sheet for the fabrication of microstructures121. Fig. 8a−c show the process of utilizing a DMD to realize layer-by-layer printing, the optical schematic diagram, and the principle of the FP-TPL system. Fig. 8d shows the resulting structures with the best-reported throughput (10-100 mm3/h) and resolution (140 nm/175 nm in the lateral and axial directions respectively). In this system, the maximum size of a single layer is 165 μm × 165 μm, and the scanning time of each layer is on the order of milliseconds; the processing speed reaches up to 1 million pixels per exposure. Compared with single-point printing, FP-TPL system improves the throughput by three orders of magnitude, making TPP suitable for large-scale fabrication applications. To the best of our knowledge, TPP-based 3D fabrication technique is the most efficient approach without having to compromise the resolution.
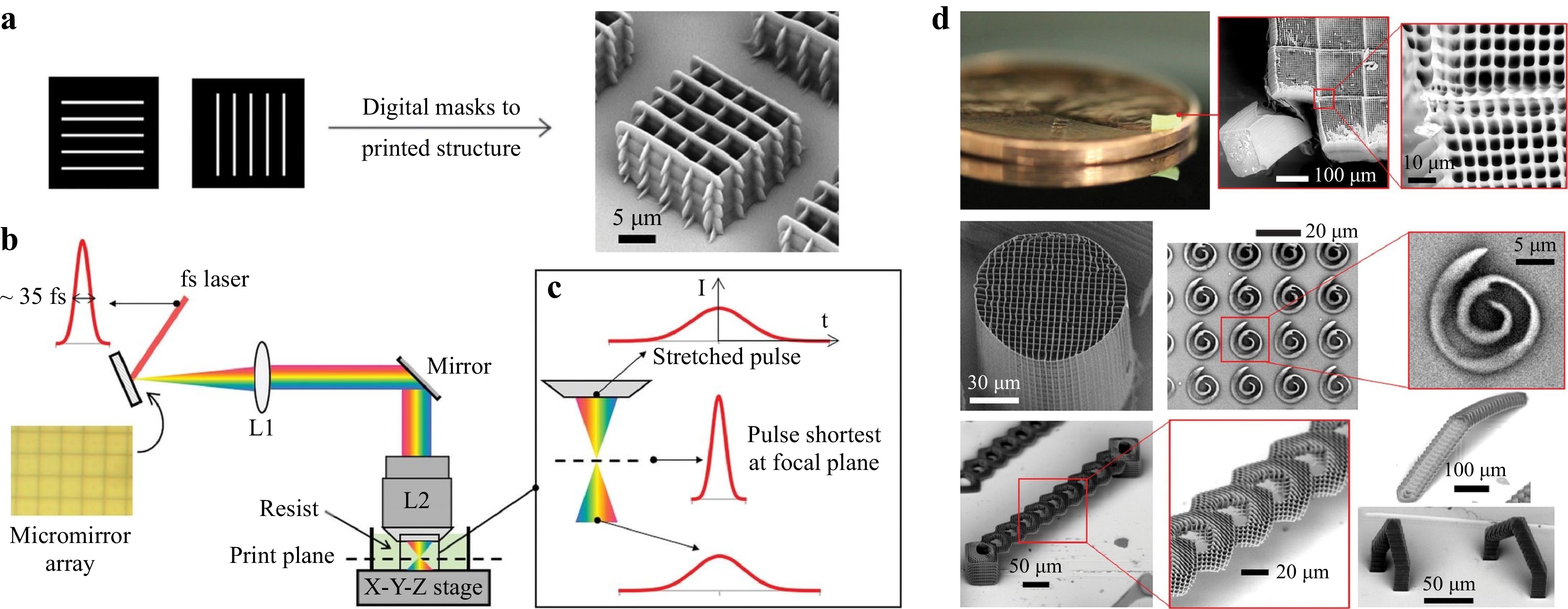
Fig. 8 a 3D printing using digital masks based with a layer-by-layer projection. b Schematic diagram of the optical path of the FP-TPL system. c Schematic diagram of temporal focusing. d The structure is obtained by processing with the FP-TPL system (reprinted from AAAS: Science121, copyright 2019).
-
Unlike point-, multi-point, or layer scanning methods, the volumetric fabrication method distinguishes itself for the control over every voxel's exposure dose or intensity in the whole 3D working space. A 3D object is formed when the power or dose at the designated position exceeds the polymerization threshold. The first volumetric fabrication method was proposed and demonstrated by Shusteff et al. in 2017122, as illustrated in Fig. 9. They utilized the holographic patterns of light fields to control the intensity in a 3D working space. The laser power was carefully controlled to cure the resin at the designated position with high intensity, thus achieving one-step volumetric AM. As shown in Fig. 9, the optical design of the printing system included a phase-only LC-SLM, which was used to generate holographic patterns in X-Y-Z directions. With two 45° prisms, the beams at the folded side and at the bottom intersected with the central beam in the working space. The region of the intersection was solidified, and thus formed a 3D part. This method achieved the fabrication of 3D structures at mesoscale with a single exposure within a duration of 5-10 seconds. Micro-3D printing can be achieved with the use of a beam delivery system of higher magnification.
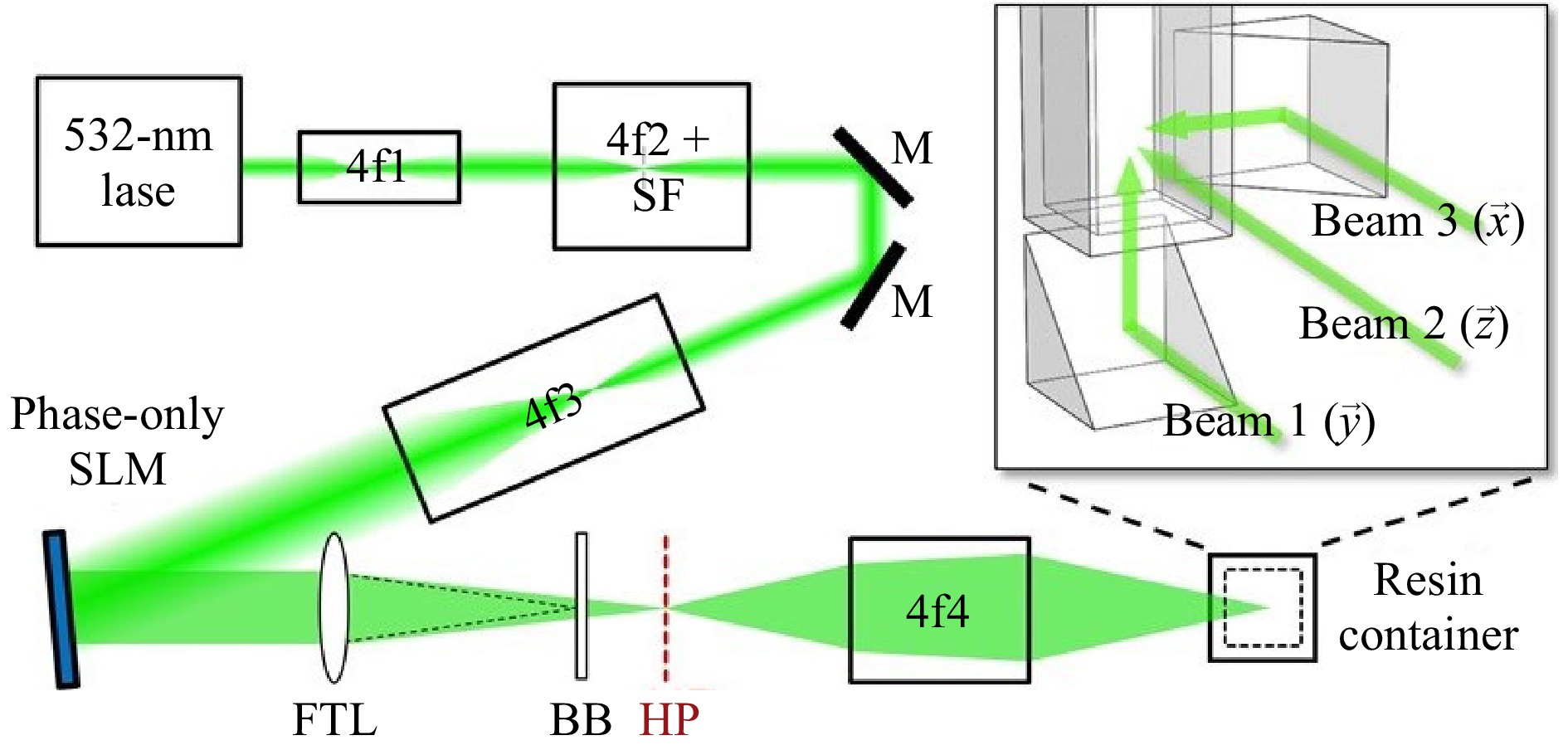
Fig. 9 Optical configuration of the holographic volumetric 3D fabrication system (reprinted from AAAS: Science Advances122, copyright 2017).
Driven by advanced microscopies, innovative volumetric additive 3D printing methods are developed, e.g., computed axial lithography (CAL). In 2019, Bhattacharya et al. proposed a volumetric AM method via tomographic reconstruction123, of which the printing mechanism and configuration are as shown in Fig. 10a, b. According to his design, the exposure dose was controlled by illuminating the resin at a constant rotation speed with a dynamic light pattern. Based on the target 3D structure, 2D images were calculated as a function of the rotation angle and projected serially to the rotating resin. Complex and support-less structures were thus fabricated with soft materials while obtaining superb surface smoothness. Attested as a high throughput approach, the CAL fabrication for centimeter-scale structures can take only 30-120 seconds. Nevertheless, the resolution remains limited at 300 μm due to the étendue of the light source in the projection process, which is unavoidable and may cause distortion to the printed object. In 2020, Loterie et al. proposed a tomographic system with higher feature resolution using a low-étendue illumination system124. They employed an integrated closed-loop feedback system, as shown in Fig. 10c, to precisely control the resin photopolymerization kinetics in the whole working space. A feedback algorithm was established to factor in the influence of light étendue, the resin viscosity and reactivity, and the tomographic reconstruction dose to achieve high resolution and fidelity. Fig. 10d shows that once the feedback was introduced, 3D structures of centimeter-scale could be fabricated within 30 seconds, while obtaining 80 μm positive and 500 μm negative feature sizes. In addition, in 2020, Regehly et al. reported an advanced volumetric AM technique, i.e., xolography, which eliminated the nonlinearities by using two intersecting light beams of different wavelengths to solidify localized regions. With such improvement, solid objects were printed with a feature resolution of up to 25 µm at a rate of up to 55 mm3/s125. The resolution was also enhanced by ten times compared with other volumetric methods, and the volume generation rate was at least three orders of magnitude higher than TPP.
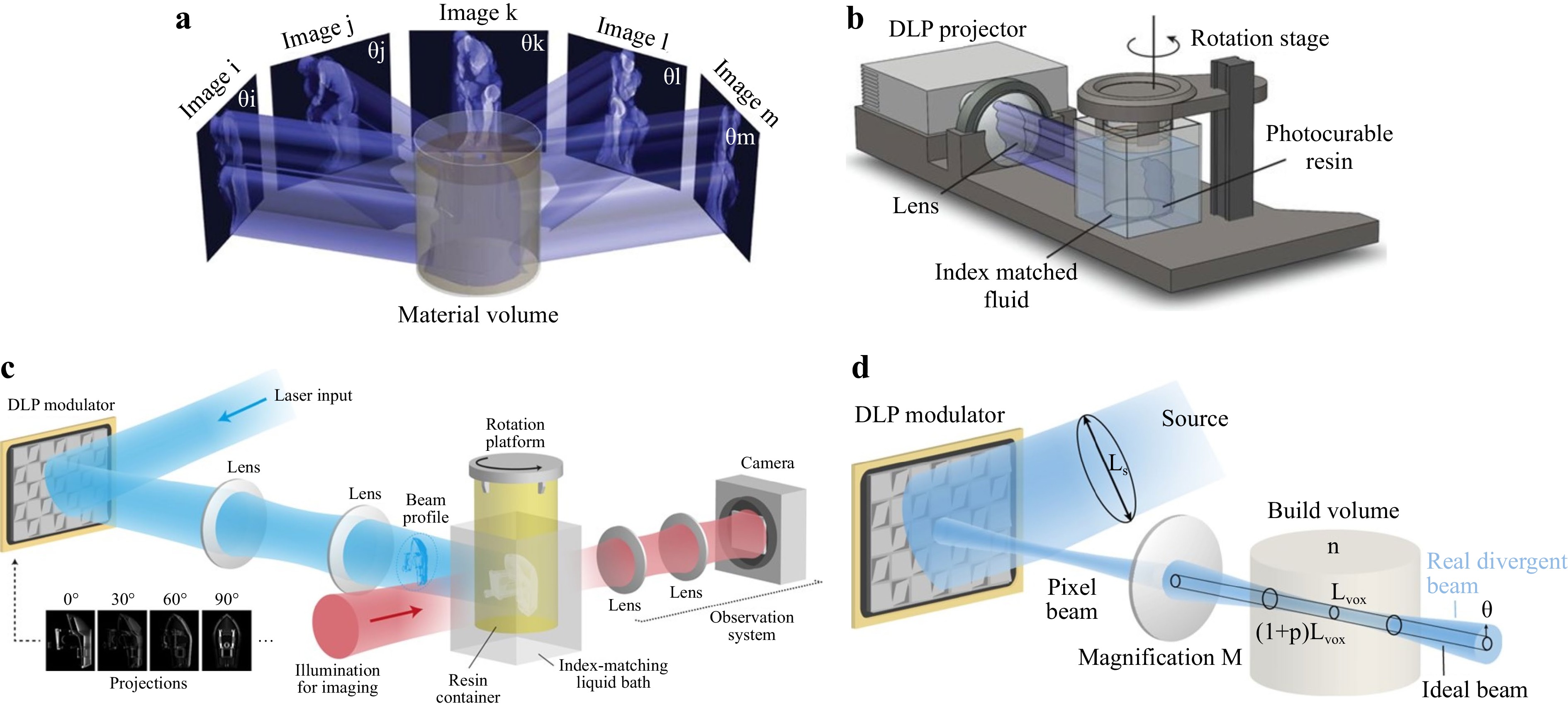
Fig. 10 a Printing mechanism of the computed axial lithography; b Configuration of the computed axial lithography (reprinted from AAAS: Science123, copyright 2019). c Configuration of tomographic 3D fabrication system with feedback; d Etendue-limited optical resolution (reprinted from Springer Nature: Nature Communications124, copyright 2020).
In comparison with point or layer scanning methods, the volumetric method excels in throughput, fidelity, and surface smoothness, but falls short in resolution due to the integration effect. Nevertheless, the volumetric approach has paved the path for ultrafast production of mesoscale 3D parts with precise structures.
-
In the TE field, the scaffold serves as a framework to support cell attachment, proliferation and migration, an extracellular matrix, and also a carrier for therapeutic cargos. Having to mimic the properties and functions of living tissues, therefore, certain criteria for scaffold design and fabrication must be factored in, i.e., biocompatibility, biodegradability, mechanical properties, biological activity, and scaffold architecture3. Otherwise, any random scaffold with uncontrollable morphology will not reproduce the complex and specified features of normal tissues as required126. In other words, one needs to have precise control over the intended scaffolds, from mimicking cellular microenvironments and regulating cell behaviors at nanometer and millimeter scales, to matching the tissue’s mechanical and biochemical properties at macroscopic scales127. However, the design based on conventional scaffold fabrication techniques, e.g., salt-leaching, gas-foaming, phase separation, or freeze-drying, is usually constrained by the partial control over the scaffold architecture128, 129. With the introduction of advanced optical methods, e.g., light-based extrusion, SLA, SLS and TPP, the scaffold fabrication has significantly improved in areas such as surface chemistry, morphology and microstructures, thanks to the enhancement in resolution, efficiency, automaticity, repeatability, and spatial control130.
In the following section, we will introduce four main types of biomaterials, i.e., metals and alloys, ceramics, polymers, and composites, as well as the applicable optical methods for scaffold fabrication.
-
Metallic and alloyed materials have long been used in TE applications, e.g., load-bearing implants for orthopedic surgeries, due to their high mechanical strengths131-133. The most widely used metal and alloy in clinical applications include iron, cobalt, chromium, stainless steel, nitinol, titanium, their respective alloys, and cobalt-chromium alloys134. Fig. 11a displays a 3-centimeter long miniaturized human femur model fabricated with Fe-30Mn powder135.
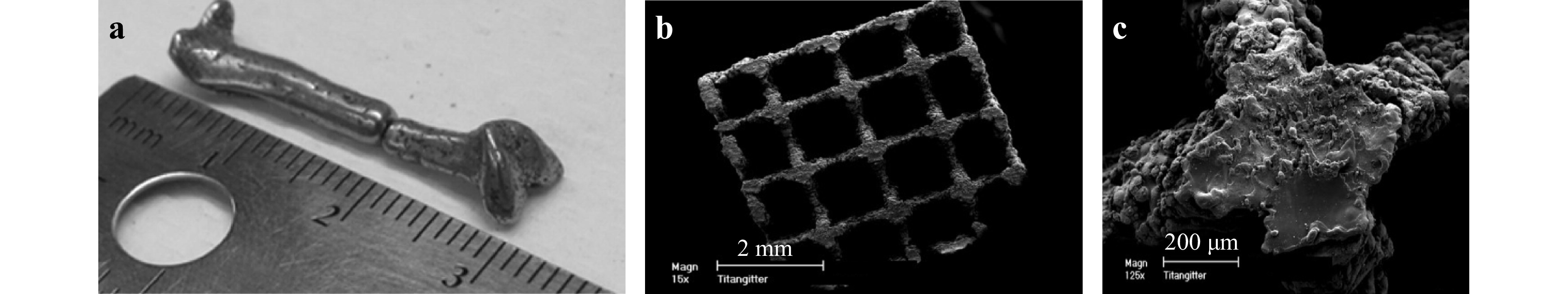
Fig. 11 a 3D printed miniaturized human femur model fabricated with Fe-30Mn powder after SLS (reprinted from Elsevier: Acta Biomaterialia135, copyright 2013). b−c SEM images of Ti6A14V scaffolds for cell differentiation and osseointegration (reprinted from Mary Ann Liebert: TE Part C: Methods136, copyright 2008).
At present, there is only a limited number of available 3D printing methods for metallic scaffold fabrications, and among which the most common ones are SLS and selective laser melting (SLM). When applying these methods, the metal material is first deposited by a nozzle or in a powder bed, and then selectively melted and fused. The printing resolution could reach tens of microns137, 138 at a printing speed of approximately 100 cm3/h137. Fig. 11b, c show an example of Ti6A14V scaffold with microrough surface resulted from the melting process, which may support cell differentiation and osseointegration136. Besides, these methods can print a wide range of materials based on powder and wire, including pure metal, alloy, and metal composites, and among which the most investigated are titanium alloys, aluminum alloys, cobalt-based alloys, nickel-based alloys, and stainless steel139.
The importance of biocompatibility and corrosion resistance of metallic implants cannot be emphasized enough, for the lack of which may lead to severe infections or even life-threatening reactions133. At the same time, these scaffolds need to be degradable so as to yield sufficient growth space for the new tissue. Therefore, biodegradable metals (BMs) are preferred because they offer desirable degradation and compatibility in vivo, and can be readily metabolized by pathways in human body. Pure metals and alloys containing magnesium (Mg), iron/Ferrum, zinc, and calcium have been used to produce biodegradable metallic scaffolds. For example, Chou et al. discovered that the iron-manganese (Fe-Mn) alloy worked well as bone scaffold materials135. They fabricated scaffolds with Fe-Mn as the powder bed via extrusion-based printing technique. An unspecified water-based organic solvent was used as the binder solution. The resulting Fe-Mn scaffold demonstrated strong tensile mechanical properties, similar to that of human bones, as well as satisfying biodegradability that promotes cell proliferation and bone regeneration18. Table 1 summarizes common metals and alloys used in TE applications. In-depth discussions on metal- and alloy-based tissue scaffolds can be found in Refs. 140-142.
Metals and alloys Applications Ref. Mg Implantation of magnesium alloy AZ91D open-porous scaffolds into the
distal femur condyle/condyles of the knee of rabbits.[143, 144] Titanium (Ti) Implantation of powder metallurgy processed Ti13Nb13Zr porous samples into rabbit tibiae [145] Tantalum Implantation of porous tantalum metaphyseal cones(Zimmer Inc. Implex, USA)into 16 patients with total knee arthoplasty. [146] Ti6A14V Implantation of porous Ti6Al4V scaffold for human osteoblasts [136] Ni-Ti Implantation of Ni-Ti porous superelastic cage in 62 patients (21 to 61 years) with total hip arthroplasty. [147] Ti-Nb-Zr-Sn Acetabular hip cup completed with complex outer scaffold. [148] Metallic nanoparticles Implantation of FeMg-NPs-containing nanoink to eradicate bone-metastatic tumor and repair the tumor-associated bone defects. [141] Table 1. Common metals and alloys for TE applications
-
Ceramics are characterized by high mechanical stiffness, low elasticity and high brittleness3. Ceramic-based scaffolds with hard tissues are exceptionally biocompatible and bioactive because their chemical structures mimic the mineral phase of native bones. In dental and orthopedic practice, ceramics are commonly used as coating for metallic implants to repair bone defects126.
There are two common types of bioceramics for scaffold fabrication: bioinert ceramic and bioactive ceramic. Bioinert ceramic materials mainly consist of alumina (Al2O3) and zirconia (ZrO2). Because of their excellent mechanical strength and corrosion resistance149, 150, alumina and zirconia have been extensively used in the fabrication of artificial femoral heads, acetabular cups, prosthetic bearings, and dental implants151. The introduction of other inert materials, e.g., titanium dioxide (TiO2) and silicon carbide (SiC)152, 153, has further prompted the clinical application, for instance, in the manufacture of micropillar array stents via inkjet printing technology152, 153. Nevertheless, Alumina and Zirconia are not bioactive despite their biocompatibility, toughness, and low friction. As a result, bioactive ceramics such as hydroxyapatite154, calcium silicate155, and tricalcium phosphate (TCP)156 with high osteoconductivity are highly demanded, as they provide mechanical support and help initiate bone generation. For example, hydroxyapatite, as the primary constituent of teeth and bones, is widely used for its capability to enhance osteoblast differentiation and proliferation154, 157-159. Fig. 12a, b are an example of such hydroxyapatite porous scaffold. Here it is essential to have precise control over the pore size and uniform distribution on the scaffold. Ceramic suspensions containing hydroxyapatite of different concentration are first used to create customized scaffolds, and once the dynamic properties are tested, the right ceramic suspension can be manufactured via SLA160.
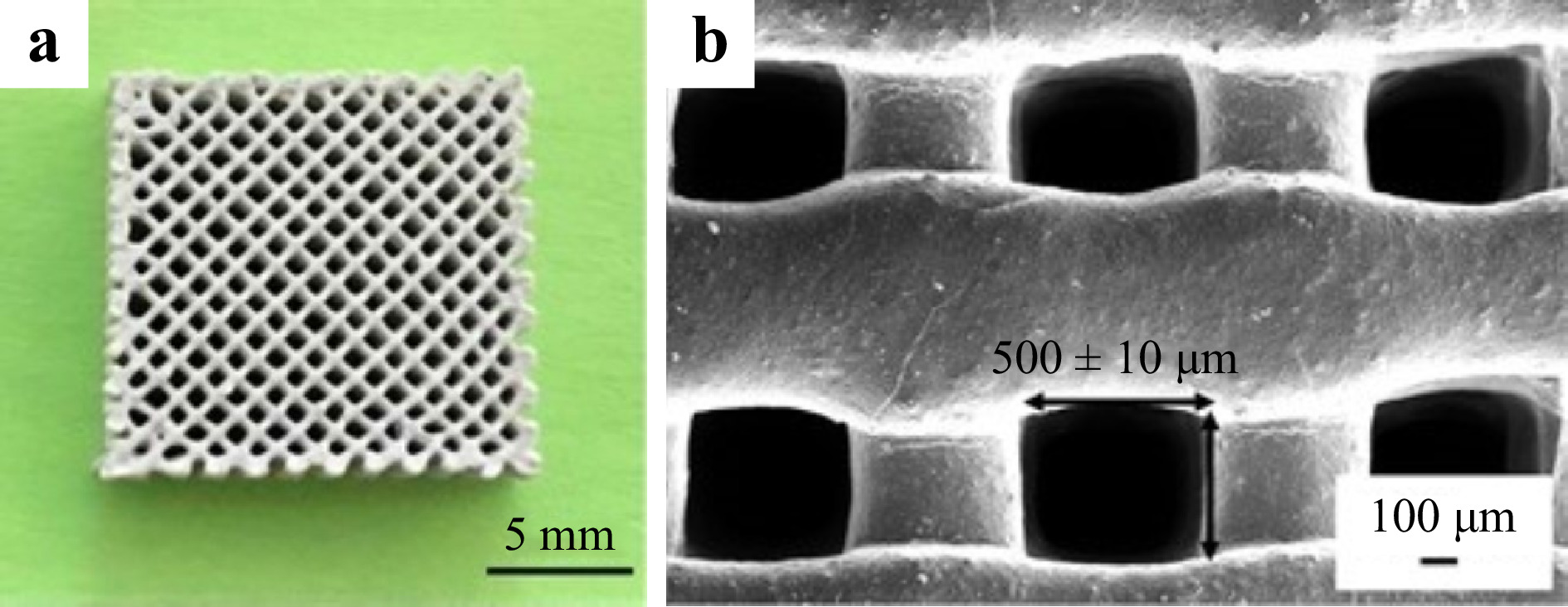
Fig. 12 a Macroscopic morphology and b SEM image of the hydroxyapatite porous scaffold (reprinted from Elsevier: Ceramics International159, copyright 2019).
The use of ceramics in TE is limited due to its brittleness, the lack of compliance with soft tissue, and uncontrollable degradation rate. To address these challenges, different 3D printing techniques have been explored to fabricate ceramic-based scaffolds with enhanced properties161, and among which SLS proves to be the most successful. Ceramic devices manufactured via SLS, e.g., customized cytocompatibility scaffolds, have been well received in the biomedical field. By optimizing the laser power, scanning speed, scanning distance, and layer thickness, such device provides more growth space for cells and higher stent carrying capacity162. DIW is another effective 3D printing technique for fabricating ceramic devices; for example, a stent was printed with concentrated ceramic paste to be later used as an active element in a polymer material163. By adjusting the size of the pores, a scaffold made by the DIW technique that matches the size of the trabecular bone is manufactured. Such approach is considered one of the best strategies for bone repair164. In-depth discussions on ceramic-based tissue scaffolds can be found in Refs. 165-167.
-
Polymers, unlike non-degradable metals and or ceramics, are widely used as source materials due to better processability. Polymers are unique in properties, e.g., biocompatibility, tissue-adaptability both in mechanical and physical aspects, biodegradability, stimuli responsiveness, and processability168, 169. As a result, polymer-based scaffolds are well adopted in in vivo environment to mimic original tissue structures and functions, and to provide cell-responsive regulations. As polymer-based scaffolds are extremely versatile, fabrication methods for natural and synthetic polymers have been extensively investigated126, 170 to respond to the demands in drug delivery, wound dressings, bone and cartilage implants, or neural regeneration171-177.
Undoubtedly, the prospect of polymers in TE application relies on successful integration of advanced optical technologies and scaffold fabrication. A recent study demonstrates that polymer crosslinked hydrogels can be bioprinted across and within the tissue of living mice by using two-photon cycloaddition, which is by far the first reported success of intravital 3D bioprinting of over 2 mm. The key to deep tissue penetration is through a precise spatial control of multiphoton microscopy and near-infrared excitation178, as shown in Fig. 13a, b. Another example is the development of minimally invasive 4D polycarbonates scaffolds via stereolithography, where the tunable shape memory effect in 3D structures is achieved179, as shown in Fig. 13c, d. Such breakthroughs demonstrate that with advanced optical methods, enhanced scaffolds for TE are achievable.
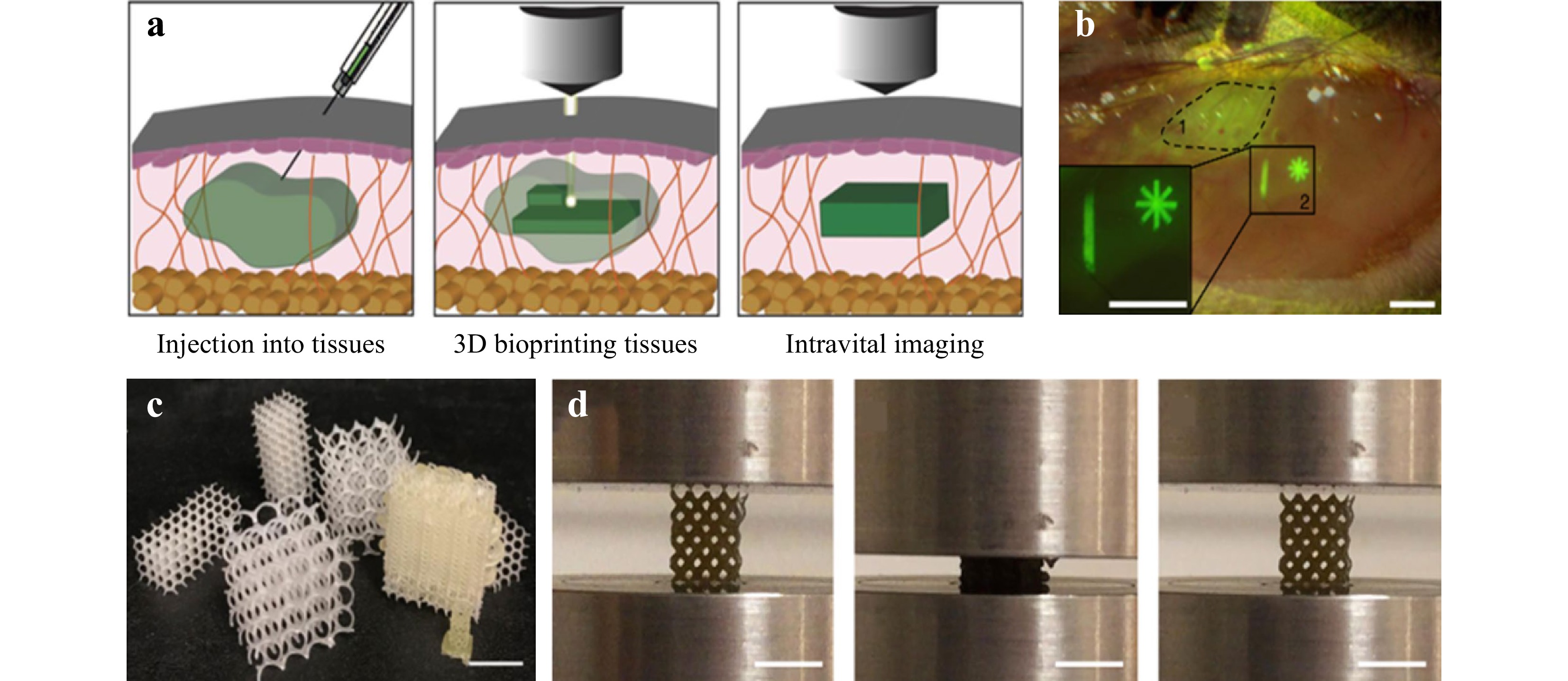
Fig. 13 Examples of the latest research progress in the TE field achieved through advanced optical methods. a Intravital 3D bioprinting is performed by injecting hydrogel precursor into live organs, fabrication of 3D hydrogel objects by two-photon excitation, and intravital imaging for hydrogel identification and in vivo analysis. b Bright-field and fluorescence stereomicroscope image of injected precursor under epimysium without (dotted line, 1) or with (continuous line, 2) hydrogel photo-crosslinking. Scale bar = 1 mm (reprinted from Springer Nature: Nature Biomedical Engineering178, copyright 2020). c 4D polycarbonates photopolymerization via stereolithography for patient-specific, self-fitting scaffolds with a wide range of surface morphologies. d 4D scaffolds display tunable shape memory with high strain recovery (reprinted from Springer Nature: Nature Communications179, copyright 2021).
-
Natural polymers used for scaffold fabrication can be classified into proteins, polysaccharides, and polynucleotides. Natural polymers such as collagen (gelatin), alginate, agarose, chitosan, and hyaluronic acid are derived from living organisms, and many of them are important ECM components3. In general, natural polymers-based scaffolds, being highly biocompatible, can reduce immune response, provide suitable microenvironment for cells, regulate critical signaling pathways and achieve positive tissue interactions180. For example, collagen is the most common protein in body and also the most critical ECM component (with gelatin as its partially hydrolyzed form, whose chemical structures are presented in Fig. 14); and it can provide cellular recognition that regulates cell adhesion, proliferation and functions. Such high bioactivity is achieved through the binding sequence (Arg-Gly-Asp, RGD) naturally existed in the polymer structures of collagen and gelatin3, 181. When collagen and gelatin are degraded by metalloproteinases in the body, new tissues are generated to replace the degraded scaffolds via cell migration182. Therefore, collagen and gelatin scaffolds are extensively utilized to regenerate bone, cartilage, nerve, muscles, and vasculature183-188. Notably, collagen and gelatin molecules can self-organize into fibrils to form hydrogels when the temperature increases to over 37 °C. This unique property gives them high printability as bioinks189. The combination of collagen and gelatin-based scaffolds and 3D bioprinting has great potential in fabricating artificial organs and tissues, e.g., skin, bone and cartilage, cardiovascular tissues, liver, cornea and nervous systems189-191.
Hyaluronic acid (HA), as a biodegradable linear polysaccharide with high hydrophilicity, is also an important ECM component (Fig. 14)192. In fact, HA’s role in wound healing, angiogenesis, and cartilage formation has been highly regarded193, owing to the ideal interaction of bioactive HA with variable surface cell receptors (such as CD44) that induce signaling pathways to direct cell fate and promote tissue formation194. For example, photocrosslinkable methacrylated hyaluronic acid (MeHA) is widely used as a bioink for 3D bioprinting. To overcome the non-cell-adhesive nature of MeHA, researchers developed a hybrid bioink combining MeHA and Methacrylated gelatin (GelMA) for SLA 3D bioprinting with enhanced mechanical strength, printability, and cell-adhesive nature195. Table 2 summarizes common natural polymers for TE applications.
Polymers Applications Ref. Collagen Regeneration of skin, bone and cartilage, cardiovascular tissues, liver, cornea and nervous systems [189] GelMA Soft tissue engineering and hard tissue engineering [191] Alginate Bioprinted hydrogels for bone, cartilage tissue engineering [196] Agarose Carriers for cell delivery and vascular engineering [197, 198] Chitosan Tissue repair and organ printing [199] MeHA Tissue engineering and regenerative medicine applications [200, 201] Table 2. Common natural polymers for TE applications
While being biologically active and biocompatible, natural polymer-based scaffolds fall short in mechanical properties, controllable degradation rate, and batch-to-batch consistency180. However, these drawbacks can be addressed by enhanced fabrication techniques such as cross-linking and hybridization126. DIW, SLA, and TPP are all common optical techniques being used in the polymerization and crosslinking process202-207, and among which the most widely used is DIW. Burdick et al. developed a hydrogel-based DIW approach that permitted the printing of HA-based shear-thinning hydrogels directly into self-healing supporting alginate hydrogels207. In addition, SLA is widely adopted to fabricate porous polymer scaffolds. For example, Lam et al. used methacrylated gelatin (GelMA) and methacrylated hyaluronic acid (MeHA) as bio-inks to mimic cartilage ECM structures via SLA bioprinting approach206. On the other hand, TPP also emerges as a laser-based technology to prepare architecturally precise polymer scaffolds to achieve higher resolution and spatial complexity than single-photon-based methods. However, TPP presents more requirements for polymerization as opposed to single-photon polymerization, despite for the fact that any photocrosslinkable scaffold systems should be able to be fabricated via TPP in theory. Without sufficient research and understanding, only a handful of natural polymers, e.g., gelatin, HA, alginate, and albumin with acrylate or methacrylate functional groups, have been reported for scaffold fabrication205, 208-211.
-
In recent years, synthetic polymers prevail over natural polymers in scaffold fabrication, mostly for better flexibility, reproducibility, processability, batch-batch consistency, and cost effectiveness212, 213. Although synthetic polymers lack biological cues inherently provided by many natural materials, modifications can be made to alter polymer moieties or introduce different functional groups to control degradation, regulate mechanical strength and enhance biological responses. By doing so, the application of synthetic polymer in biomedical field is considerably broadened213, 214.
Poly(ethylene glycol) (PEG), also known as poly(ethylene oxide) (PEO) is one of the most common synthetic polymer materials in TE, as shown Fig. 15215. Being approved by FDA, PEG is highly hydrophilic, flexible, soluble and biocompatible, thus suitable for hydrogel fabrication via variable crosslinking methods with conjugated end groups of carboxyl, thiol, or acrylate216. One should bear in mind that PEG and other synthetic polymers such as poly(vinyl alcohol) (PVA) and poly(acrylic acid) (PAA) are non-degradable; therefore, modifications (e.g., by conjugation of enzymatically degradable peptide) may be required216. On the other hand, poly(α-hydroxy acids), including poly(lactic acid) (PLA), poly(glycolic acid) (PGA), poly(caprolactone) (PCL) can be degraded by hydrolysis217; however, these polymers displayed extremely low hydrophilicity, which is disadvantageous for TE application where wetting ability in vivo, cell attachment and tissue interaction are critical214.
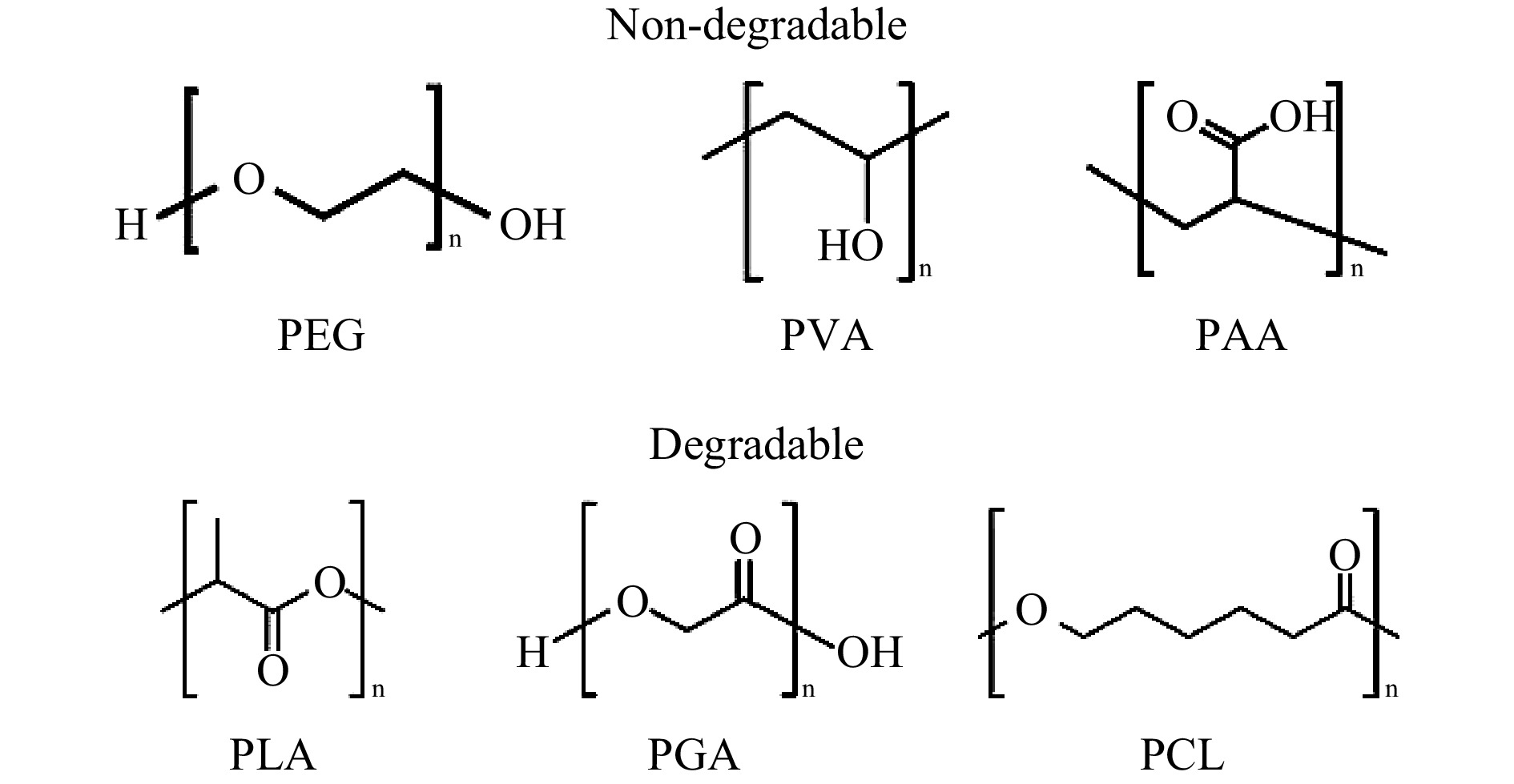
Fig. 15 Chemical structures of typical degradable and non-degradable synthetic polymers used for scaffold fabrication.
Advanced optical methods are gaining popularity in the fabrication of synthetic polymer-based scaffold181, 203-205. As one of the most common synthetic polymers in TE, PEG is widely adopted to fabricate hydrogels via TPP to control the spatial, mechanical, temporal, and biochemical architecture of scaffolds178, 211, 218-223. Recently, Gao et al. reported a poly(ethylene glycol) diacrylate (PEGda) hydrogel via TPP that displayed low cytotoxicity. In their study, a new kind of ionic carbazole water-soluble photoinitiator was used to achieve a low printing laser threshold of 3.7 mW and a high resolution of 180 nm. In general, the fabrication of hydrogels via TPP underlines the importance of two-photon photoinitiators with good solubility and high two-photon absorption (TPA) cross-section in the design211. However, a recent study reports the use of a photosensitive PEG hydrogel crosslinked by initiator-free two-photon cycloaddition to avert toxic-level concentration of photoinitiators and the release of a large number of radicals into cells and tissues178. Such finding will certainly propel the research in the application of radical-free and initiator-free TPP technologies. Table 3 lists selected examples of synthetic polymers used in TE applications.
Polymers Applications Ref. PGA Mesh networks for musculoskeletal, cardiovascular, vaginal, intestinal, lymphatic, and spinal regeneration [224] PPF Scaffolds in bone TE [225] PEG Scaffolds for musculoskeletal, vascular, dental pulp, and endothelial tissue regeneration [226] PVA Fabrication of scaffolds for cartilage-like tissue [226] PPE Biocompatible and biodegradable scaffolds for cartilage tissue [227] PPZ Biodegradable scaffolds for hard TE applications [228] Table 3. Applications based on synthetic polymers.
-
Although the scaffold function can be enhanced with the use of metal, ceramic or polymer, each of these materials has its own defect that limits a more comprehensive application, e.g., metal toxicity, brittleness of ceramics, and low strength of polymers. As such, researchers start to investigate different composite combinations so as to acquire the best implant solution, and it is at this point when composites come into play for scaffold fabrication via 3D printing with enhanced mechanical strengths and intricate details.
Composite scaffolds can demonstrate mechanical controllability229 and compressive strengths as needed in the implant site, as well as enhanced bioactivity230-232. In fact, composites are primarily used in musculoskeletal TE, mainly bone repair, where mechanical controllability and customizability are of critical importance. The most common composites contain hydroxyapatite, TCP, or bioactive glass particles or fibers used as fillers or coatings or both in PLA, PGA, or other resorbable polymers233, 234. For example, by adding hydroxyapatite into PLLA and PLGA, Tang et al. fabricated scaffolds for bone tissue that demonstrated better osteoconductivity, superior buffering capability and improved mechanical properties229. Shapiro et al. found that the composite hydrogels consisting of ceramics, for example, maintain a hydrophilic polymeric network that mimic the innate tissue, and possess good mechanical strengths to withstand the compression forces caused by cell proliferation and differentiation233. In fact, composites are possibly the most vital biomaterials for 3D scaffold fabrication. Table 4 lists selected examples of composite biomaterial components used in TE applications234.
Bio-composite Type Characteristics Application Young’s modulus Metal and alloy Cobalt
based alloysMechanical properties Neurosurgical and vascular implant fabrication, fracture fixation implant 190 − 253 GPa235 Nitinol
(Ni-Ti alloys)Shape memory effect,
pseudo elasticityDental, orthopedic, cardiovascular uses 28 − 41 GPa (Martensite)
75 − 83 GPa (Austenite)236Austenitic stainless steel Reasonable strength,
fatigue resistanceVascular stent and electrode cardiac pacing system 200 GPa237 Ceramics Bio-glass, and
glass ceramicsPorosity and bioactivity Bone defect 35 − 118 GPa235 Zirconia
(ZrO2)Mechanical properties,
fracture toughnessTotal hip replacement, ball head 200 GPa238, 239 Natural
polymersCollagen Protein in abundance, low antigenicity, bio-compatible
mechanical propertiesScaffold for soft tissue repair 0.35 MPa240 Silk fibroin Mechanical properties,
bio-compatibilityScaffold for soft tissue repair 23 KPa (collagen)
50 KPa (chitosan)241Alginate Bio-compatible,
nontoxic, biodegradable, hydrogel formationScaffold for cartilage repair 25 KPa242 Synthetic
polymersPolyethylene glycol (PEG) Higher permeability to gases, nutrients, and metabolites, biocompatibility Drug delivery,
scaffold fabrication470 MPa243 Polycaprolactone Slow biodegradable,
structural flexibility, nontoxic metabolism of its degraded productsScaffold for cartilage and bone matrix 2.4 GPa244 Table 4. Characteristics and TE applications of composite biomaterials234.
-
Traditionally, the study of basic life science has been profoundly benefitted from in vitro cell studies performed in 2D cell culture systems245. Nevertheless, the morphology and behavior of the cell growth on flat 2D surfaces is fundamentally different from those in living tissues246, hence the concern over the accuracy and validity of these studies. As a result, biomaterial based 3D scaffolds have been developed in recent years to mimic the complex 3D tissue architecture, reflect real cell activities in ECM and enable natural intercellular interactions. Optical-based 3D printing is considered promising for customized 3D scaffolds for cell studies247. As mentioned in Section 2, specific 3D printing technique is selected as per the requirement and purpose of cell studies in order to fabricate scaffolds with desired compositions, morphologies, biochemical properties, and mechanical strength.
SLA is a common technology for 3D cell culture scaffold fabrication. When combined with computer-aided design, SLA can fabricate controlled and defined scaffold geometries with high resolution18, 248. For example, Chen et al. demonstrated the use of SLA technique through mixing hydroxyapatite powder into photosensitive resin to fabricate 3D scaffold. Such scaffolds exhibited non-cytotoxicity and excellent biocompatibility249. SLS techniques have also been employed to prepare scaffolds from bio-compatible and bio-degradable polymers such as polyetheretherketone, polycaprolactone, and polyvinyl alcohol. Liu et al. proposed a bio-composite slurry consisting of hydroxyapatite, silica sol, and sodium tripolyphosphate to generate scaffolds using SLS250. These scaffolds demonstrated mechanical strength as high as 43.26 MPa, but with low porosity.
Extrusion-based printing systems, which deposits materials precisely onto a surface to form defined structures, are highly flexible in fabricating 3D scaffolds in a single-step process246. Materials are deposited in a layer-by-layer fashion where each layer may contain a combination of different materials. In general, commercial extrusion-based printers perform the printing of cell-laden gels, often with other polymeric materials such as metallic material and hydrogel, to yield viable and functional scaffolds18. In 2021, Putra et al. reported that extrusion-based 3D printing could fabricate scaffolds with porous iron with enhanced biodegradability and mechanical properties, a bone substituting biomaterial with great potentiality251.
In addition to the abovementioned optical methods, TPP’s precise and independent control of the chemical, mechanical, and geometrical properties in a printed object enables the creation of well-defined extracellular environments. For example, Ricci D et al. developed a cell culture substrate via TPP. Fig. 16 presents micro-fabricated scaffolds containing nichoid structures for the cell growth. Fig. 16a, b present a 200-µm side triangle and 300-µm side hexagonal nichoid structures with high resolution. Fig. 16c, b) present fluorescent images of human bone marrow-derived mesenchymal stem cells (stained with DAPI) seeded and cultured on the triangular and hexagonal scaffolds. Owing to unique scaffold design, the experiments demonstrated the best reported culture surface coverage rate of 88%252.
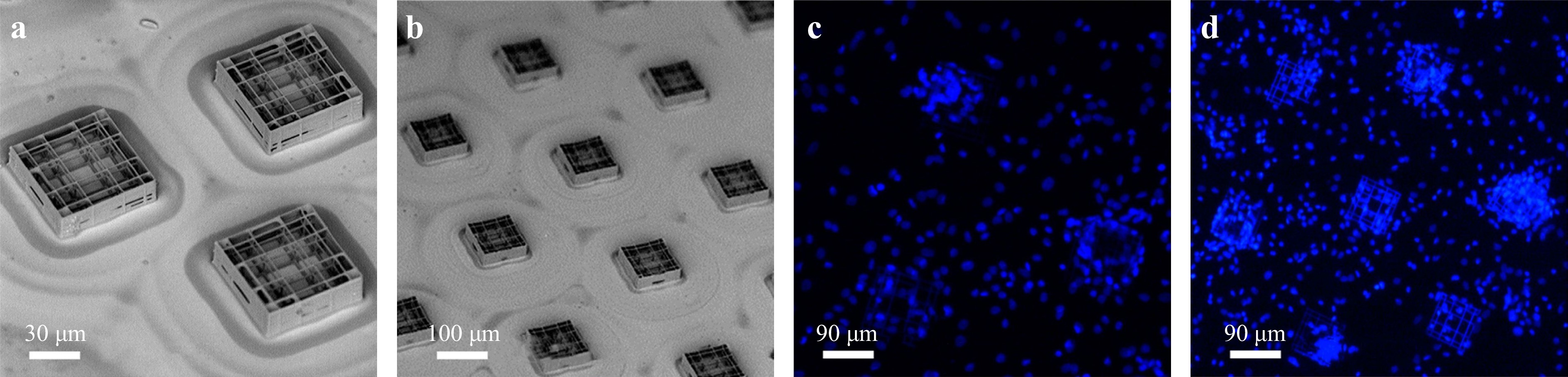
Fig. 16 SEM images of the culture substrate composed of nichoids patterned in a a 200-µm side triangle; b 300-µm side hexagonal layout. c and d Fluorescence images of cell-populated substrates. Elementary nichoids patterned in a c 200-µm side triangle; and d 300 µm side hexagonal layout. (reprinted from MDPI252, copyright 2017).
Advanced optical methods can influence cell culture not only through dimensionality but also through dynamicity. PDMS scaffolds fabricated via TPP is one example in the study of tumor cell invasiveness. Spagnolo et al. fabricated micro scaffolds (a volume of about 50 × 50 × 50 μm3) with two different pore sizes, as shown in Fig. 17a, b253. They used TPP to control the stiffness of microenvironments and to induce local structural variations by modulating the laser power during lithography (Fig. 17a, b), thus permitting the observation of MCF cell invasion mostly driven by soft architectures, as shown in Fig. 17c, d. Such application in architecture design via 3D printing has greatly benefitted the study in cell dynamics253. Recently, Rovira et al. examined different TPP design parameters to produce cell-like polyacrylate scaffolds with specific properties. These scaffolds were modified with a lipid bilayer supported on a cationic polymer to mimic human cell membranes, generating even lower critical dimensions to approximately 200 nm. Their work proved that with the selected photoresists, printing parameters, post-processing methods, greater design freedom and high resolution could be obtained at the same time, thus enabling the fabrication of biomimetic cell membranes that mimic both the shape and mechanical properties of human cells254.
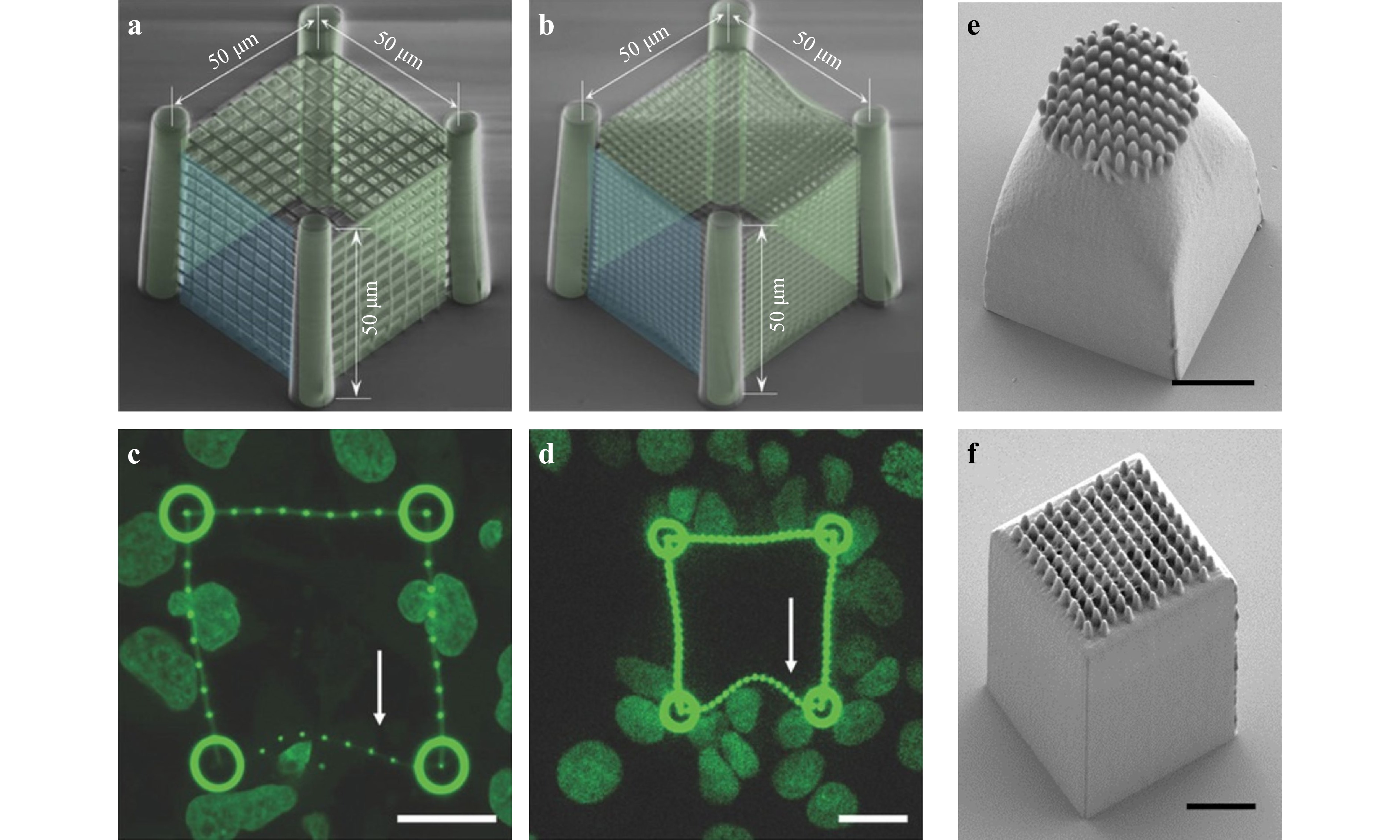
Fig. 17 Cancer cells migrating through a porous cubical scaffold. a−b Representative colored SEM images of two different pore sizes. c−d Being invaded by MCF7, cells with low stiffness facet (as indicated by the arrows) deform by the cells pushing against the cage. Scale bar: 20 μm (reprinted from Wiley: Advanced healthcare materials253, copyright 2017). e−f Micrographs of columnar epithelial cell scaffolds printed using two configurations, scale bar: 5μm (reprinted from Elsevier: Materials & Design254, copyright 2021).
-
Drug delivery refers to the integration of approaches, systems, technologies, and formulations that act as a medium of transport to administer therapeutics into the body to exert therapeutic effects255, 256. The introduction of optical 3D printing methods and their applications have induced the rapid development of drug delivery technologies, from oral dosage delivery to the delivery of small drug molecules to biodegradable scaffolds for large molecules255. The main driving force for the rapid development lies within 3D printing’s capability to fabricate drugs of customizable size, shape, and dose with precise release profiles at low cost and shortened cycle time. In this section, we will introduce a number of preeminent applications of drug delivery via optical 3D printing methods.
In 1996, Wu et al. first demonstrated the fabrication of biodegradable implants to exhibit the feasibility of drug delivery257. In recent years, optical based 3D printing methods have been increasingly used in pharmaceutical applications. For example, via an extrusion-based 3D printer, Khaled et al. fabricated a quick delivery tablet loaded with 80% paracetamol258. In 2020, Cui et al. fabricated a fast-release formulation of levetiracetam based on semi-solid extrusion printing technology achieving a loading content of 96%259. Laser-based 3D printing methods, such as SLA and SLS, have also been extensively applied in drug delivery applications. For example, Robles-Martinez et al. fabricated “polypills” that contain six active ingredients with unique drug release profiles via an SLA printer260. Economidou et al. fabricated a microneedle array via SLA using biocompatible resins for transdermal insulin delivery261. In summary, the use of 3D printing technology in drug delivery system, especially in the application of bone TE, has been well studied in the last few decades, and the tremendous progress is proof of its potentiality. Table 5 provides a list of these applications. More details on drug delivery can be found in a separate review Ref. 262.
Ref. Material 3D printing Drug Applications [262] Hierarchical 3D-multidrug scaffolds based on nanocomposite bioceramic and PVA with Gel-Glu external coating Extrusion Dipyridamole and BMP-2 Bone TE [263] PCL 3D printing patient-specific implant, degradable porous silicon-based carriers SLA BMP-2 Bone graft for critical-sized bone defects. [264] Poly(3-hydroxybutyrate) scaffold. SLS Osteogenic growth peptide and its C-terminal sequence. Bone TE. [265] MBG is functionalized with polydopamine and PGPL. SLS Dexamethasone Osteogenic differentiation and biomineralization. [266] Mesoporous iron oxide nanoraspberry inside microneedles. DLP Minoxdil Treatment of androgenetic alopecia. [267] Porous poly(ethylene glycol) dimethacrylate devices. TPP Rhodamine Different biomedical applications. Table 5. Applications of 3D printing-based drug delivery.
-
Bone TE consists of scaffolds, seed cells, and cytokines. The process to repair bone defects begins with the transplantation of tissue scaffolds to the defective area, followed by the replacement of scaffold material with the growth of new bone tissues268-270. An ideal bone scaffold for clinical application should meet the requirements for porosity, surface area ratio, mechanical support, biocompatibility, surface activity and shape, and capability to promote cell adhesion and blood vessel and nerve growth6, 271. Fig. 18 shows the use of 3D printing in different TE applications, which exploits a wide range of materials, e.g., metals, polymers, ceramics, or cells encapsulated within a bio-ink. One can see that 3D printing is most widely used in bone and cartilage, accounting for approximately 31% of overall application. Extrusion-based printing, a popular technique, fabricates scaffolds with bio-inks which is a mixture of one or multiple biomaterials with live cells270. As a form of bio-ink, hydrogels are typically used for printing cells, morphogens, and growth factors for bones and cartilages. Different formulations exist to make bio-ink hydrogels, including the seven different hydrogel formulations proposed by Bendtsen et al. to find the optimal composition to produce bone tissue scaffolds272.
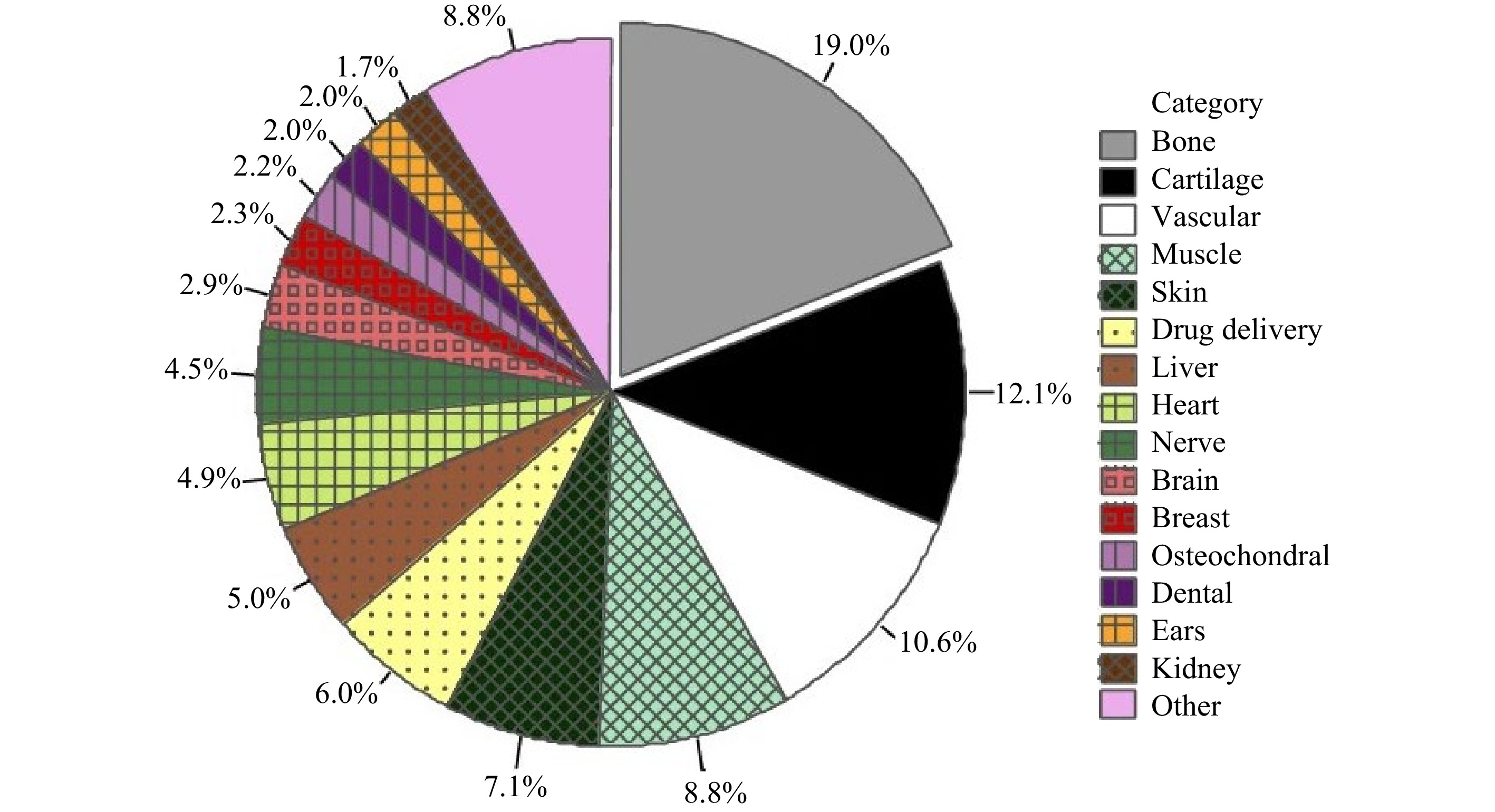
Fig. 18 Use of different 3D printing approaches in bone scaffold fabrication (reprinted from Springer Nature: BioMedical Engineering OnLine270, copyright 2020).
In addition to the extrusion-based method, SLA and SLS are proven successful in building bone tissue scaffolds from photo-cross-linkable poly (propylene fumarate) (PPF). Such scaffolds can be cross-linked through its carbon-carbon double bonds, and degraded in the body by simple hydrolysis of the ester bonds into nontoxic products273, 274. As suggested in the study by Lee et al., on a conventional SLA machine, the UV curable polymer resin composition and laser parameters must be optimized to fabricate 3D scaffolds with controlled microstructures for specific bone TE applications. Moreover, to achieve the desired pore size and porosity, both in vitro and in vivo biological evaluations are critical for scaffold fabrication via SLA274. Roskies et al. used SLS technology to create a customized porous polyether ether ketone scaffold that maintained the viability of adipose and bone marrow mesenchymal stem cells and induced osteogenic differentiation of adipose-derived mesenchymal stem cells275. To satisfy the specific requirements for osteochondral repair, Du et al. constructed a bioinspired multilayer osteochondral scaffold consisting of poly (ε-caprolactone) and hydroxyapatite microspheres via SLS. These SLS-derived scaffolds (4 mm in diameter, 2.8 mm in thickness) exhibited excellent biocompatibility to support cell adhesion and proliferation in vitro276.
TPP combined with the use of biodegradable polymers has great prospect in the area of micro-structured scaffold fabrication for bones. Recently, scaffolds based on cross-linked biodegradable polymers, including copolymers of lactide and e-caprolactone, have been regarded as a reliable technique for future rapid prototyping in the medical field208, 277. For example, Timashev et al. applied TPP to fabricate hexagon-shaped scaffolds from synthetic biodegradable star-shaped polylactide (SSL) materials. These TPP-fabricated SSL scaffolds demonstrated a high Young's modulus value (> 4 GPa) with densely cross-linked structures and high spatial resolution. When loaded with human adipose-derived stem cells in vitro, the scaffold displayed excellent in vivo biocompatibility with no obvious sign of necrosis, inflammatory responses and fibrous membrane formation210. In 2017, Maggi et al. fabricated 3D rigid polymer scaffolds with tetrakaidekahedral periodic geometry, whose strut dimensions were on the same order as osteoblasts' focal adhesions (2 μm) and pore sizes on the order of a cell (~10 μm)278. A thin layer of TiO2 was subsequently coated to ensure biocompatibility and stiffness (in the range of 0.7 – 100 MPa). Osteoblast-like cells (SAOS-2) were on these scaffolds (Fig. 19), and finally, the effectiveness was verified by tracking mineral secretions and intracellular fraction and vinculin concentrations of the cell growth after 2, 8, and 12 days in the mineralization media.
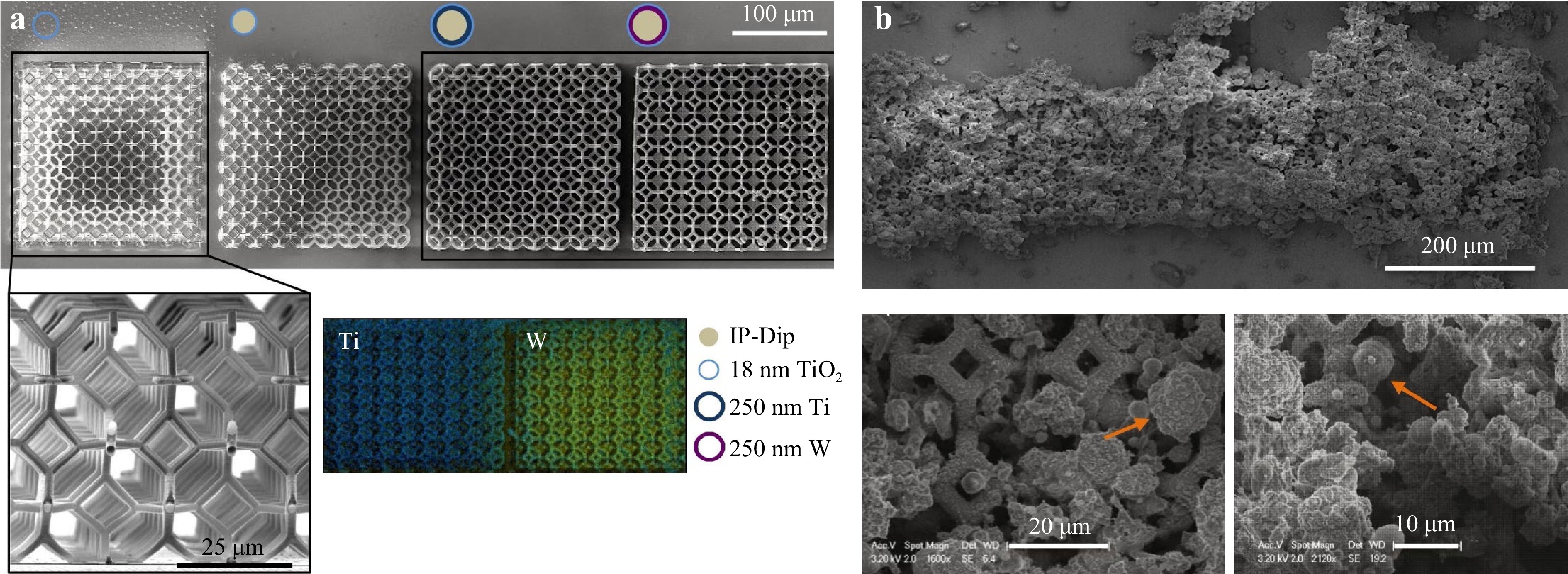
Fig. 19 a SEM view of the fabricated samples. b SEM images of the samples after 8 days (top) and 12 days (bottom) of growth. Mineral deposition (orange arrow) on the lattice scaffolds by Saos-2 cells (reprinted from Elsevier: Acta Biomaterialia278, copyright 2017).
In addition to photoresist, TPP can also fabricate structures via hydrogels with high resolution and spatial complexity, which makes TPP an emerging optical process to prepare scaffolds for bones and cartilages205, 209. For example, Felfel et al. fabricated well-defined multiphase scaffolds for bone repair via TPP by recombining hydrogel and hydroxyapatite nanoparticles. These scaffolds exhibited instantaneous shape recovery after being compressed, which is critical for arthroscopic delivery279. Without doubt, selecting the most suitable material for TPP process is essential to 3D scaffold fabrications due to its considerable impact on the viability and behavior of bone cell and tissue. For example, bovine chondrocytes adhere easily to woodpile scaffolds made from certain organic-inorganic hybrid materials; they either lay or migrate on top of the scaffold or completely infiltrate into the scaffold, depending on the pore size of the woodpile205, 209. Also, it is found that certain urethane dimethacrylates and gelatin meth acrylamides facilitated the adhesion of bovine chondrocytes on rectangular TPP-based lattice scaffolds, whereas PEGDA, with minimal cellular behavior differences, significantly prevented cell adhesion.
-
Angiogenesis and vasculogenesis are two mechanisms through which blood vessels are formed. The sprouting blood vessels grow into the ischemic tissue in response to attractive or repulsive biochemical signals, mechanical cues, and the gradients in tissues. An interconnected vascular network is required to maintain the viability and biological function of a large growing cell population. Scaffold-based tissue regeneration, particularly with large and thick scaffolds, necessitates the incorporation of interconnected vascular network between the cells within scaffolds and the culture medium or blood, in order to facilitate the mass transfer of nutrients, signaling molecules, oxygen, growth factors, metabolic waste, etc., which manifests the core concept of TE280, 281. Fig. 20a, b present two examples of 3D printed scaffolds for vascularization and micro-vascularization.
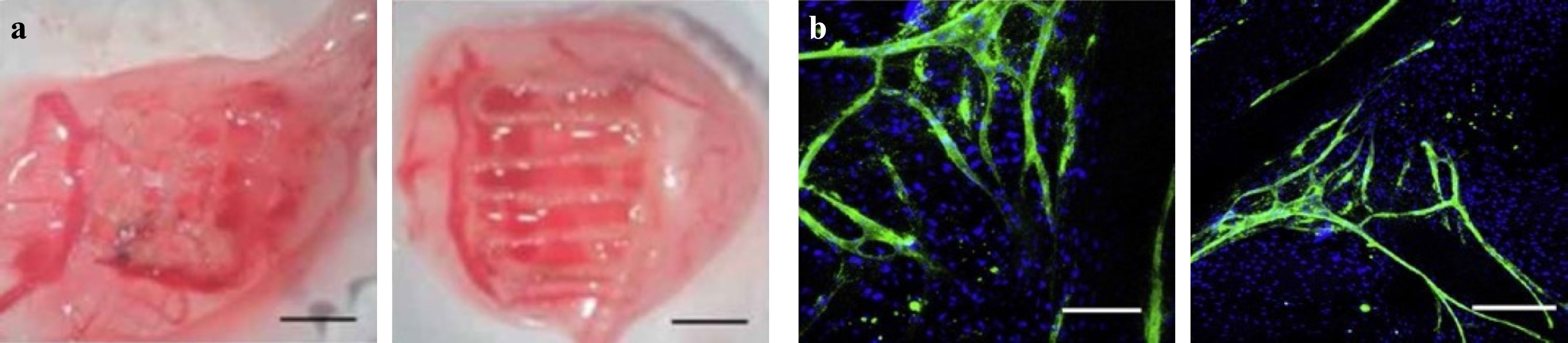
Fig. 20 a Example of 3D printed scaffold vascularization (reprinted from IOP: Biofabrication282, copyright 2020). b Distribution and organization of human dermal microvascular endothelial cells and with primary human osteoblasts on fiber-mesh scaffolds (reprinted from Elsevier: Biotechnology Advances283, copyright 2009).
With regard to scaffolds for angiogenesis, biomaterial-based scaffolds fabricated via extrusion-based printing is the most prevalent. To encapsulate the vascular network and the scaffold, hydrogels or cell/peptide-loaded hydrogels are cross-linked by UV light. An example worth mentioning is the osteon-like scaffold which can be manufactured via extrusion-based 3D bioprinting to enhance the generation of cardiovascular systems284. From the in vitro culture study, it is found that proper cell orientation and scaffold structure can promote the generation of new blood vessels. For cardiovascular and bone regeneration, the optimal distance between cells has been found by optimizing the scaffold structure285; and scaffolds containing closely connected pores with a size exceeding 250 µm are essential characteristics of the stent286, 287.
Thanks to the recent advances in photolabile polymer, scaffold fabrication can be also realized via SLA/SLS approaches to address the issue of nozzle clogging and bio-ink viscosity, and ensure high-resolution printing with outstanding accuracy. For example, SLA techniques can fabricate large scaffolds with detailed vascular networks in a layer-by-layer fashion with the use of photosensitive materials and photoinitiators13, 288. Nevertheless, laser-based methods, despite of their capability to create 3D structures with speed and precision, may cause cytotoxicity if inappropriate materials are used; therefore, they should be applied with caution and when the use of advanced biomaterials with biocompatibility is in presence.
Soft tissues connect and support surrounding tissues or organs, e.g., tendons, ligaments, blood vessels, muscles, fat, fascia, synovial membrane, and nerves. Common scaffold materials for tissue repair and regeneration are synthetic or natural polymers. Moreover, synthetic materials continue to evolve so as to ensure that biological tissues maintain their specific functions and inherent mechanical strengths. Synthetic hydrogel is a type of biomaterial suitable for manufacturing biological scaffolds due to its adjustable mechanical properties and biodegradability289, 290. As tissue differs in size and shape, such flexibility is ideal for making soft tissue engineered scaffolds. One should bear in mind that other conditions need to be fulfilled to achieve flexibility and processing accuracy, e.g., scaffold porosity’s essential role in controlling biological functions. Though achieving precise control over shape and porosity may be challenging via traditional molding methods, micro-level accuracy with stereolithography 3D printing is still attainable while simultaneously satisfying the chemical, biological, and mechanical properties in customized products67, 291. A typical example of soft tissue reconstruction is oral soft tissue reconstruction, where individual oral defects can be addressed via the integration of 3D printing technology and computer-aided design.
Most common scaffolds used for soft tissue healing are hydrophilic polymers based because of their capability in forming well-defined 3D matrices that mimic the microstructure and physicochemical properties of ECM224. As such, the cells are exposed to an appropriate level of biomechanical stimulation, thus enhancing their physical integration into the highly hydrated body environments such as musculoskeletal, myocardium, cornea, and other soft tissues226, 292, 293. In fact, when targeting soft tissue scaffolds, SLA technique prevails optical extrusion-based or SLS methods for its capability in printing flexible and soft materials. For example, Hockaday et al. fabricated highly accurate aortic valve geometries by using SLA with PEG and alginate294. These scaffolds exhibited a sizeable elastic modulus range (about 5.3 ± 0.9 to 74.6 ± 1.5 kPa), accurate shape dimensions, and high viability for encapsulated porcine aortic valve interstitial cells295.
On the other hand, one should also pay attention to the biocompatibility and toxicity issues in TE, which can be crucial, for example, when cells, biochemicals, and tissue scaffolds are simultaneously processed or printed; during such process, the effect of heating, pressure, and light-matter interaction processes can potentially influence the designed experiments adversely or sometimes favorably296. Recent studies on toxicity and biocompatibility of materials in TE can be found in Refs. 296-300.
-
In the previous sections, we reviewed the 3D scaffold fabrication in TE from the perspectives of methods, materials, and applications. Table 6 shows the comparison across various 3D printing methods as per their respective advantages, disadvantages, fabrication rate and resolution. Fig. 21 illustrates some of the exemplary works and their respective performances. Based on these facts, we summarize and conclude that:
Methods Materials Rate Resolution Advantages Disadvantages Applications in TE Extrusion/
jetting
basedPolymers,
Heated thermoplastic materials4000 cm3/h310 ~ 100 μm ▪ Simple operation
▪ Versatile applicable materials
▪ Adaptable for bioprinting▪ Potential toxicity
▪ Time consuming (Postprocessing)18Soft tissue311
Vascular repair312
Drug delivery262
Vascular scaffolds284SLA Photopolymer,
(Liquid)103 − 106 mm3/h122 10 − 150 μm313
(2 μm for LAPμSL78)▪ Complex internal features
▪ High resolution67
▪ Large part bioprinting
▪ Smooth surface finish▪ Photopolymer required314
▪ Support structure required
▪ Small volumeBone tissue273, 274.
Drug delivery263, 266
Vascular
networks13, 288SLS Polymer, Ceramic,
Metal and alloy
(Powders)50 − 200 μm
(5 μm for μ-SLS90)▪ High mechanical properties
▪ No need for support structures
▪ Fine resolution
▪ Excellent control over microstructures▪ Limited material selection (must be shrinkage and heat resistant)
▪ High temperature required (1.4k°C)250
▪ Time consuming and material wastageDrug delivery264, 265
Bone scaffolds275, 276
Osteogenic differentiation265TPP Photopolymer, Hydrogel Up to 10 −
100 mm3/h121Up to ~100 nm ▪ Very high resolution
▪ Excellent flexibility in 3D structures▪ High cost
▪ Limited material selectionBone and cartilage209, 278.
Soft tissue283
Angiogenesis and vasculogenesis282,
Drug delivery267Volumetric Photopolymers Up to 103 − 105 mm3/h122, 125 25 − 500
μm125, 29▪ High fabrication rate ▪ Lower resolution
▪ Limited material selectionBeing realized Table 6. Comparison of different optical-3D printing methods.
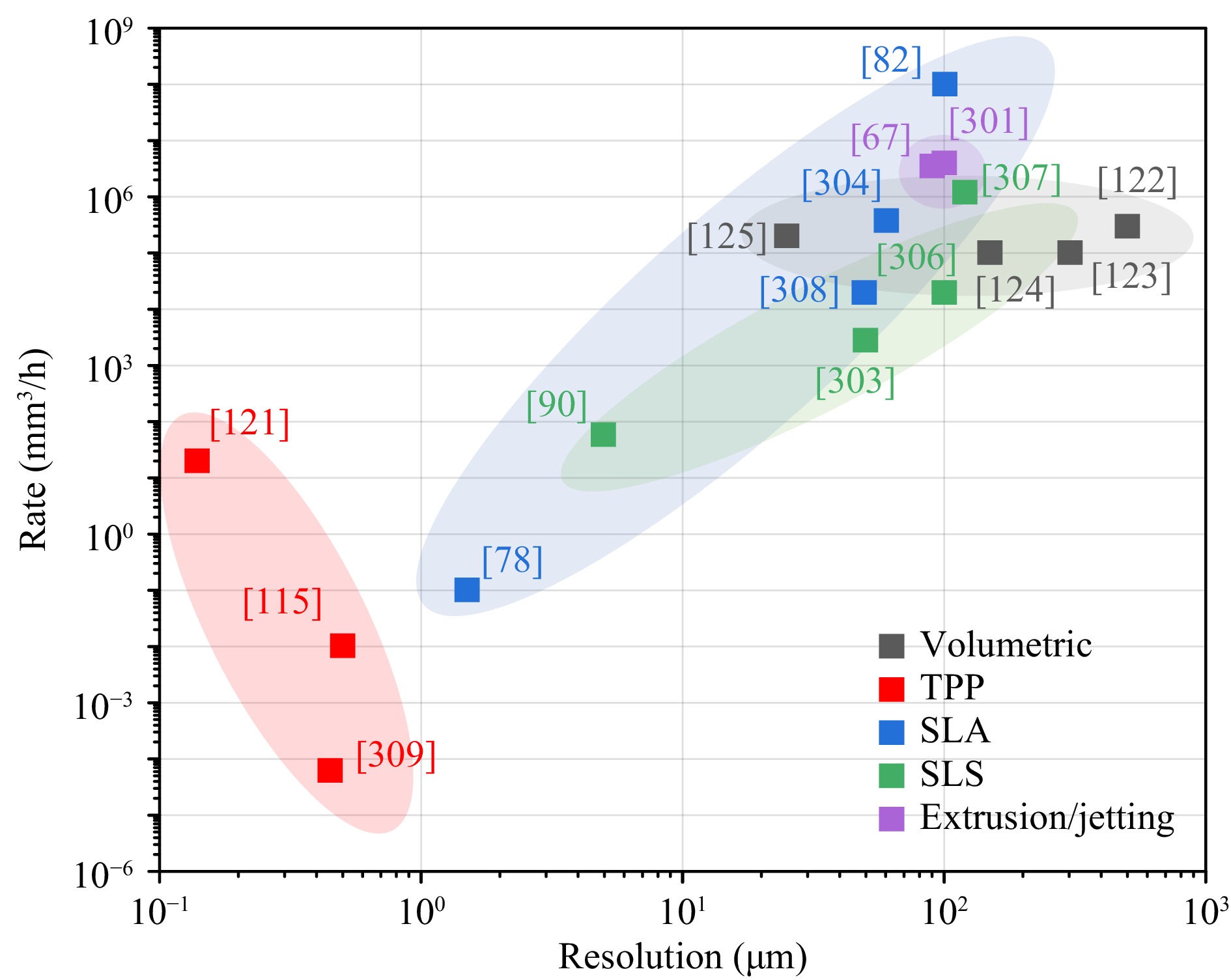
Fig. 21 Summary of different optical 3D printing methods plotted in fabricate rates versus resolution. The labels of the data point refer to the serial numbers of the corresponding references.
The extrusion/jetting based optical 3D printing method distinguishes itself in simplicity and the applicability to a wide range of biocompatible materials with outstanding throughput and fabrication rate67, 301. It is arguably the most straightforward solution to scaffold fabrication; however, the resolution at hundred-micron level rules out the possibility of fabricating scaffolds with finer structures.
With better fabrication rate and resolution, SLA and SLS are two optical methods that dominate the present market despite of the limited resolution at micron scale78, 82, 87, 90, 302-308. SLA and SLS can be used to fabricate 3D scaffolds with a wide range of material options including those in liquid and solid states.
The TPP method, despite of slightly slower fabrication rate (10-100 mm3/h), enjoys the best resolution, which reaches a hundred nanometers or higher115, 121, 309, as well as the capability to print arbitrary 3D structures. As such, TPP technique has the greatest potential in fabricating small-scale scaffolds with complex structures. Since the range of applicable materials for TPP is limited, further studies in advanced materials and their properties are of critical importance for future advancement.
The volumetric printing method sets itself apart with fabrication rate as high as liters per hour122-125. Nevertheless, the approach is hindered by poor resolution and limited material selection. According to the literature, the volumetric printing method is suitable for fabricating scaffolds with pore size of several hundred microns.
-
This paper presents an overview of the latest optical 3D printing methods that revolutionize the scaffold fabrication in TE from the perspectives of fundamental concepts, materials, and potential applications. We also examine the fabrication performance in terms of precision and fabrication rate under various scenarios, followed by recommendations for future studies in the fields of optics and TE. Optical 3D printing methods are extremely effective due to superior performance and cost-effectiveness, and the prospect of broader application depends on the breakthrough in new materials that address the fast-growing demand in 3D scaffold fabrication in TE. In fact, we recognize a positive interplay among scaffold applications, materials and 3D printing methods. In other words, the demand for advanced scaffold has been the driving force for the development in material and 3D printing methods and vice versa. Going forward, the topics of interests for future studies may include but not limited to:
-
The fabrication of large-scale 3D scaffolds remains quite challenging, as optical 3D printing methods, especially TPP, achieves a much higher resolution (up to hundred nanometers) at the expense of printing time and ultimately the scaffold's final size. In a recent publication, Weisgrab et al. reported a TPP-based regenerative medicine scaffold with a large volume of 292 mm3, yet in a processing time of 16 hours315.
Materials and optical systems are two key elements to achieve high throughput and printing rate. First, a fast processing speed depends on photoinitiators with a high two-photon absorption cross-section (δ, with the unit of Göppert Mayer (GM)). Typical one-photon photoinitiators merely have δ values on the order of around 20-3049, while those explicitly developed for TPP can reach 150315. Therefore, processing speed can be improved by developing highly efficient photoinitiators materials, and also a lower initiator concentration is preferred when fabricating biocompatible scaffolds.
Moreover, advancement in optical systems, particularly with the introduction multiple foci and volumetric fabrication, can greatly enhance the efficiency. Gittard et al. introduced an effective scaffold fabrication by TPP with multiple foci via SLM technique, as shown in Fig. 2250, 110. Later, Zandrini et al. combined TPP with fast linear stages to produce multi-foci and thus significantly reduce the processing time by a factor of nearly five110, 224. Finally, the FP-TPL technique developed by our team is capable of printing 3D structures with the highest throughput (10−100 mm3/h) and resolution (140/175 nm in the lateral/axial directions) ever reported and a 90% cost reduction (~US$ 1.5 /mm3) comparing with current commercial solutions110, which may address the long-standing challenges in high-resolution large-scale scaffold fabrication.
Fig. 22 Scaffolds made by TPP with a single focus structuring and b four foci structuring. c Image of bovine endothelial cells growing on a scaffold made by multibeam TPP (reprinted from OSA: Biomedical Optics Express50, copyright 2011).
-
In order to further enhance the ability to mimic the complexity of ECM’s physical and biochemical properties, the integration of advanced materials and methods for scaffolds need to be developed as a resort to satisfy the requirements for multi-compositional and multi-functional microstructures.
As indicated in the past research, optical-based 3D printing methods is capable of printing multi-material systems in which each material corresponds to distinct chemical, biological, and optical properties. For example, Klein et al. reported the first multi-material printing protocol to fabricate 3D cellular scaffolds with two materials to either promote or inhibit cellular attachments24. On such basis, other researchers have developed methods to print multi-material 3D microarchitectures, including cellular environments, composite metamaterials, and optical components123, 316.
However, printing with multiple materials is exceedingly more time-consuming and labor-intensive than printing with a single material due to a more sophisticated setup. To be more specific, a printer operator’s skill may determine the registration accuracy for each additional material. In other words, while a multi-material system is designed with extraneous alignment structures and tolerances, the print-to-print repeatability still depends much on the user’s performance; hence a higher probability of failure than that of a single-material system316. One solution worth exploring is to integrate a microfluidic device with TPP printers. As such, it is possible for one to fabricate multi-material and multi-functional scaffolds, and to host several cell types in accordance with the complex tissue conditions. Furthermore, by integrating actuator systems with scaffolds with multi-functional sensors, i.e., advanced fluorescent microscopy and computational imaging methods, one can also greatly enhance the analytical capabilities of fundamental and applied researches205.
As this review covers some, if not all, of the exemplary works in the fields of 3D printing scaffolds in TE, we look forward to seeing more studies on relevant topics from both the optical and engineering communities. We believe that both the academic and industrial communities can thrive on a broadened and deeper understanding in optical 3D printing methods and its impact on scaffold fabrication in TE.
-
Table of abbreviations Abbreviation Description 3D Three-dimensional ABS Acrylonitrile butadiene styrene Al2O3 Alumina AM Additive manufacturing BMs Biodegradable metals CAL Computed axial lithography DIW Direct ink writing DLP Digital light procession DMD Digital micromirror device ECM Extracellular matrix ECM Extracellular matrix FDM Fused deposition modeling Fe-Mn Iron-manganese FFD Fused filament deposition FP-TPL Femtosecond projection two-photon lithography GelMA Methacrylated gelatin GM Göppert Mayer HA Hyaluronic acid HOTP Holographic optical tweezing process IR Infrared ISA3D Immersed surface accumulation-based 3D LAPμSL Large area projection micro-stereolithography LED light-emitting diode LCD Liquid-crystal display LC-SLM Liquid crystal-based spatial light modulator LCVD Laser Chemical Vapor Deposition LECP Laser-enabled electrochemical printing LIFT Laser-induced forward transfer MeHA Methacrylated hyaluronic acid Mg Magnesium MJ Material jetting NIR Near-infrared NP Nanoparticle PAA Poly(acrylic acid) PBF Powder bed fusion PC Polycarbonate PCL Poly(caprolactone) PED Precision extrusion deposition PEG Poly(ethylene glycol) PEG Polyethylene glycol PEGda Poly(ethylene glycol) diacrylate PEO Poly(ethylene oxide) PET Polyethylene terephthalate PGA Poly(glycolic acid) PJP Polyjet printing PLA Poly(lactic acid) PPF Poly (propylene fumarate) PVA Poly(vinyl alcohol) PVC Polyvinyl chloride PμSL Projection micro stereolithography SiC Silicon carbide SLA Stereolithography SLS Selective laser sintering SLM Selective Laser Melting SSL Star-shaped polylactide SSTF Simultaneous spatial and temporal focusing TCP Tricalcium phosphate TE Tissue engineering TiO2 Titanium dioxide TPA Two-photon absorption TPP Two-photon polymerization UCNP Up-conversion nanoparticles ZrO2 Zirconia -
This work was supported by the Innovation and Technology Commission (ITC) (ITS/178/20FP) and Centre for Perceptual and Interactive Intelligence (CPII) Ltd under the Innovation and Technology Fund.
Advanced optical methods and materials for fabricating 3D tissue scaffolds
- Light: Advanced Manufacturing 3, Article number: (2022)
- Received: 05 October 2021
- Revised: 27 March 2022
- Accepted: 01 April 2022 Published online: 06 May 2022
doi: https://doi.org/10.37188/lam.2022.026
Abstract: Three-dimensional (3D) printing, also known as additive manufacturing (AM), has undergone a phase of rapid development in the fabrication of customizable and high-precision parts. Thanks to the advancements in 3D printing technologies, it is now a reality to print cells, growth factors, and various biocompatible materials altogether into arbitrarily complex 3D scaffolds with high degree of structural and functional similarities to the native tissue environment. Additionally, with overpowering advantages in molding efficiency, resolution, and a wide selection of applicable materials, optical 3D printing methods have undoubtedly become the most suitable approach for scaffold fabrication in tissue engineering (TE). In this paper, we first provide a comprehensive and up-to-date review of current optical 3D printing methods for scaffold fabrication, including traditional extrusion-based processes, selective laser sintering, stereolithography, and two-photon polymerization etc. Specifically, we review the optical design, materials, and representative applications, followed by fabrication performance comparison. Important metrics include fabrication precision, rate, materials, and application scenarios. Finally, we summarize and compare the advantages and disadvantages of each technique to guide readers in the optics and TE communities to select the most fitting printing approach under different application scenarios.
Research Summary
3D printing: Advanced optical methods that enable new tissue scaffolds
With overpowering advantages in printing efficiency, resolution, and a wide selection of applicable materials, optical 3D printing methods have undoubtedly become the most suitable approach for scaffold fabrication in tissue engineering. Shih-Chi Chen from the Chinese University of Hong Kong presents an overview of the latest optical 3D printing methods that revolutionize scaffold fabrication in tissue engineering from the perspectives of fundamental concepts, materials, and potential applications. This review summarizes and compares the advantages and disadvantages of each technique to guide readers in the optics and tissue engineering communities to select the most fitting printing approach under different application scenarios.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article′s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article′s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.





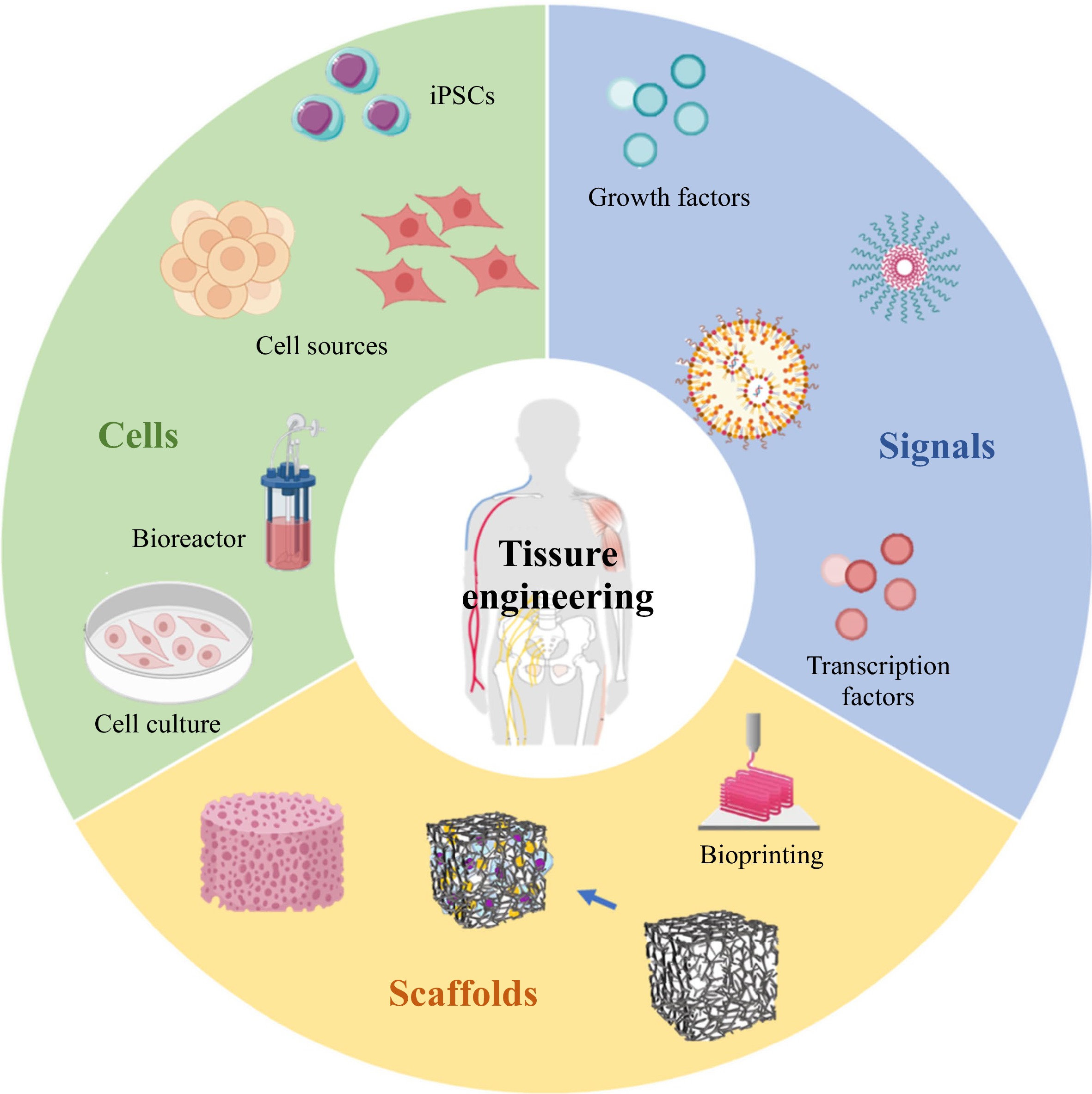
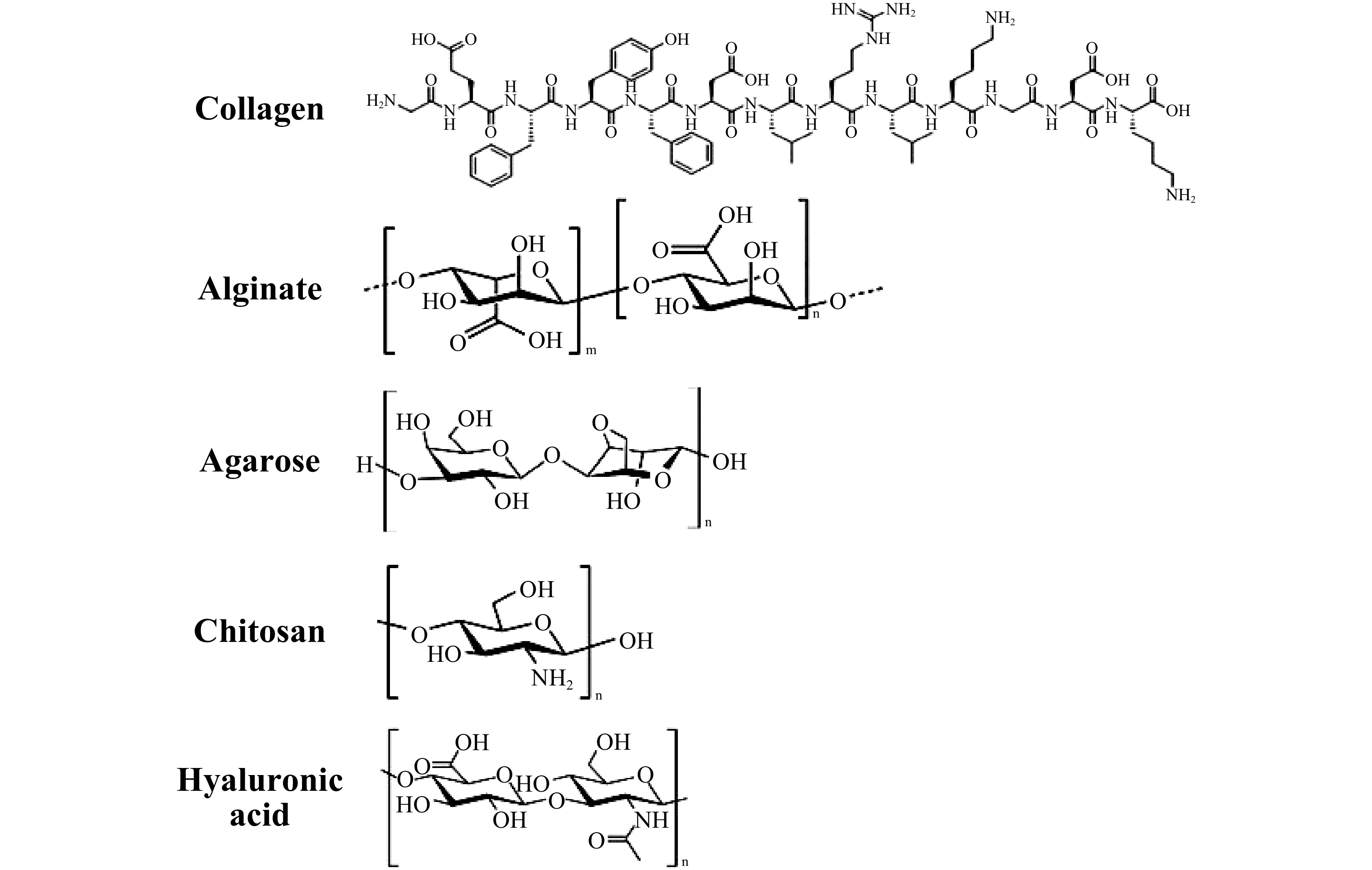

 DownLoad:
DownLoad: